
The appropriate treatment for elderly gastric cancer patients
Gastric cancer has a peak incidence between 50 and 70 years of age, and has demonstrated a rising incidence in the elderly as life expectancies have increased (1-4). Current treatment guidelines and the standard of care are commonly based on studies and clinical trials of younger patients and need to be assessed for their direct applicability to the elderly population, both in the context of their increased comorbidities as well as potential differences in their disease pathophysiology (1,5,6). This manuscript examines the current state of scientific knowledge in the management of gastric cancer and presents the evidence from the National Cancer Database (NCDB), with a special focus on the important variations and considerations that need to be made for elderly patients.
Incidence and epidemiology
Gastric cancer is the fifth most common cause of cancer and the third leading cause of cancer-related death worldwide (7,8). The incidence and mortality of gastric cancer is disproportionately varied by geographic region, with a higher rate in Eastern Asian countries and a relatively lower rate in Western countries (9,10). In Eastern Asia, gastric cancer of the distal portion of the stomach is more common, while proximal gastric cancer is more often seen in the West (3,11).
Several environmental and lifestyle factors contribute to an increased risk of gastric cancer. Helicobacter pylori (H. pylori) infection is a major cause of gastric carcinogenesis with potential progression to gastric cancer, and is also responsible for the disproportionately high prevalence of gastric cancer in Asian countries (12,13). In Japan and Korea, the substantial decline of H. pylori infection in recent decades has been accompanied by a corresponding decrease in gastric cancer incidence and mortality (14-18). The decline of gastric cancer mortality in these countries can also be attributed to the rigorous screening programs that have been implemented, as more cancers are being diagnosed at an earlier stage (19-22). Dietary and lifestyle factors such as high sodium intake, increased alcohol consumption, and smoking have also been associated with an increased risk of gastric cancer (23-27). Diets high in fruit and vegetable consumption have shown a protective effect against gastric cancer (28-30).
The elderly and gastric cancer
Currently, there is no standard definition of “elderly”. Many groups and organizations, including the World Health Organization, have used a chronological age of 65 years and older to define the elderly population; however, as the average life expectancy in many countries are reaching, and at times exceeding, 80 years of age, this definition is rapidly shifting (31,32). As a result, there are also no standard guidelines for the management and treatment of the elderly with gastric cancer.
Several differences have been identified in the presentation and pathologic characteristics of gastric cancer when diagnosed in the elderly compared with younger patients. As expected, elderly patients typically have more medical comorbidities and higher American Society of Anesthesiologists (ASA) classification scores (33-35). Elderly patients also tend to present with symptomatic disease of more advanced clinical stage (33,36,37). The primary tumors tend to be found in the distal third of the stomach (38-41). Histopathologically, elderly patients tend to have tumors that occur in multiples, of larger size, and of well-differentiated and intestinal-type histology (1,33,36,38,42-46). Several studies have also identified potentially different patterns of lymph node metastases in elderly gastric cancer patients compared with younger patients; however, the results are conflicting as some studies have demonstrated a higher propensity of lymph node metastases in the elderly, some have shown a lower propensity, and others have shown no difference (1,33,38,41,42,47-50). The interpretation of these varying findings remains difficult as the elderly often undergo a limited lymphadenectomy compared to the extended D2 lymphadenectomy which is the standard of care among younger patients. We examined 13,836 patients who underwent radical gastrectomy between 2010 and 2014 using NCDB, of which 2,140 patients (15%) were aged ≥80 years. Patients age older than 80 tended to have larger tumors and more lymph node involvement, hence with higher American Joint Committee on Cancer (AJCC) stages compared to younger patients (P<0.0001). These observations are consistent with previous reports.
Surgery in the elderly
The extent of resection is determined by the primary tumor stage and nodal status. The AJCC TNM classification is one of the most commonly used staging criteria (51). T1a tumors, defined by invasion of the lamina propria or muscularis mucosa, are typically amenable to endoscopic resection. For stage IB–III disease with a primary tumor that that invades into and beyond the submucosa but does not violate the visceral peritoneum or adjacent structures, a partial gastrectomy with extended D2 lymphadenectomy is recommended to achieve a curative resection of all microscopic and macroscopic disease (52).
As gastrectomy is a significant abdominal operation, elderly patients must be carefully evaluated preoperatively given their increased risk of morbidity and mortality with surgery. Fujiwara et al. compared 115 patients aged ≥80 years with 333 patients aged ≤79 years, who underwent subtotal or total gastrectomy with varying extent of lymphadenectomy, and found that patients aged ≥80 years had more post-operative complications and in-hospital mortality, as well as lower overall survival (OS) and disease-specific survival (DSS) (53). Using a time-dependent receiver operating characteristic curve analysis, the optimum age cut-off identified in this study for gastrectomy to produce a survival benefit at three years was 79.2 years. Hsu et al. evaluated the outcomes of 164 patients ≥80 years who underwent subtotal or total gastrectomy with D2 lymphadenectomy in comparison with 2,258 younger patients <80 years and also identified a significantly higher morbidity (18% vs. 13%, P=0.035) and in-hospital mortality rate (7% vs. 3%, P=0.015) in the elderly group (35). However, unlike the previous study, Hsu et al. did not identify any significant differences in long-term disease-specific deaths (44% vs. 47%, P=0.407) after a median follow-up of 37.8 months. Applying an even higher age cut-off to define the elderly population, Endo et al. compared 56 patients ≥85 years old who underwent distal gastrectomy with 55 patients <85 years old who received best supportive care only using propensity score-matched analysis (54). A survival benefit of gastrectomy over best supportive care in this most elderly group was demonstrated among female patients only (median OS 67 vs. 12 months, P<0.0001), but not in male patients (median OS 13 vs. 18 months, P=0.037). Post-operative pneumonia, especially among male patients, was a common complication in this study population and was often associated with mortality, which has also been reported in other studies (42,53,55-58). The gender-based survival difference in patients with post-operative pneumonia has also been identified in other elderly and highly comorbid patient populations, and is potentially a result of interactions between various hormonal, immunologic, and microbiologic factors (59-63). The overall high incidence of post-operative pneumonia in the elderly is likely due to the baseline decreased pulmonary function and reserve, which is then further diminished after a major open abdominal surgery (64-69). Non-pulmonary post-operative complications as well as post-operative death after gastrectomy have also demonstrated increasing incidence with increased patient age (34,45,70-72). These studies indicate the importance of pre-operative risk assessment and patient selection among elderly patients being considered for open gastrectomy.
One technique to reduce the post-operative morbidity and mortality among elderly patients undergoing gastrectomy is to use a minimally-invasive approach. Several studies have demonstrated comparable and, at times even improved, outcomes in elderly patients undergoing laparoscopic or robotic gastrectomy compared to an open approach (73-76). The measured outcomes in these studies included intra-operative blood loss, time to first flatus, time to first oral diet, index length of hospital stay, post-operative complications, and survival. The minimally-invasive approach produces a smaller physiologic insult on the body, with fewer long-term functional impairments (77,78). Unlike prior studies in open gastrectomy where elderly patients consistently had higher rates of post-operative complications than non-elderly patients, several studies have found the complication rates between elderly and non-elderly patients to be similar with the minimally-invasive technique (45,79-83). We have recently conducted our own review of post-operative outcomes in patients aged ≥80 years who had undergone minimally-invasive compared to open subtotal or total gastrectomy using the NCDB. We found that minimally-invasive gastrectomy was associated with decreased length of stay of at least 1 day (P<0.001) compared to open gastrectomy. In addition, there was no difference in the rate of margin-positive resections (P=0.27), adequate lymph node sampling defined as ≥15 lymph nodes (P=0.08), readmissions (P=0.32), or 30- or 90-day mortality (P=0.75, P=0.82) between these two approaches. A minimally-invasive approach to curative resection in the elderly should be highly considered to promote post-operative recovery and improve patient outcomes.
The extent of lymphadenectomy during gastrectomy and the potential survival benefit of extended D2 lymphadenectomy in elderly patients has also been controversial. While D2 lymphadenectomy, which includes nodes along the left gastric, common hepatic, celiac, and splenic arteries in addition to the perigastric lymph nodes removed in D1 lymphadenectomy, is typically recommended for stage IB–III gastric cancers, the oncologic benefit must be balanced with the potentially increased morbidity and mortality of this more extensive procedure in an elderly population with more medical co-morbidities and less functional reserve. Brenkman et al. reviewed 2,387 patients <75 years of age and 1,377 patients ≥75 years who had undergone curative subtotal or total gastrectomy and found that a high lymph node yield improved survival in both age groups, with no increase in postoperative mortality (43). However, the difficulty in interpreting this and similar studies lies in the use of lymph node yield as a measure of the extent of lymphadenectomy. Lymph node yield only records the number of lymph nodes harvested intra-operatively, but may not always accurately convey if nodes were sufficiently taken from all the necessary stations that define a D2 lymphadenectomy. In the Dutch trial of 1,078 patients who were randomized to D1 or D2 lymphadenectomy, patients >70 years consistently had higher morbidity and mortality compared to patients ≤70 years, and these rates were even higher among those >70 years who underwent D2 compared to D1 lymphadenectomy, with limited survival benefit (84). Rausei et al. reviewed 1,322 patients who had undergone curative gastrectomy with D2 versus D1 lymphadenectomy and, in addition, to categorizing patients by age (<70 versus >70 years), also stratified patients by their comorbidities using the Charlson comorbidity score (<5 vs. >5) (85). Overall, more post-operative complications occurred in patients >70 years (34% vs. 28%, P<0.001), and within this elderly population, those with high Charlson scores had an even higher complication rates (38% vs. 31%, P=0.007). Patients >70 years with high Charlson scores also trended towards having more complications after D2 lymphadenectomy, but this was not statistically significant (40% vs. 35%). Additional studies have also demonstrated the lack of survival benefit conferred by extended D2 lymphadenectomy compared to a more limited D1 lymphadenectomy in elderly patients (46,86-88) (Table 1). Our study from the NCDB suggested that elderly patients might not derive the same survival benefit from D2 lymph node dissection as younger patients. Of the 2,140 patients aged ≥80 years who underwent subtotal or total gastrectomy, half had less than 15 lymph nodes examined and half had 15 or more lymph nodes examined. Using this as a proxy for extent of lymph node dissection, there was no difference in OS between the elderly patients who had a limited compared to extensive lymphadenectomy (median OS 18.2 vs. 19.2 months, P=0.29).
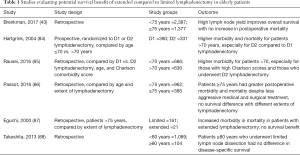
Full table
Neoadjuvant therapy for the elderly
For patients with ≥T1b tumors, neoadjuvant chemotherapy with a platinum/fluoropyrimidine combination is recommended (52). The MAGIC trial demonstrated a significant improvement in both overall (5-year 36% vs. 23%) and progression-free survival (PFS) (HR 0.66; 95% CI: 0.53–0.81) for patients with resectable gastric cancer treated with three cycles of pre-operative and three cycles of post-operative epirubicin, cisplatin, and fluorouracil compared to those who underwent surgery alone (89). Additional trials have supported the survival benefit of this neoadjuvant chemotherapy combination in patients with resectable gastric cancer (90,91). However, many the patients in these trials were young or middle-aged, with a limited number of elderly patients, especially those ≥80 years. Trials focusing specifically on elderly patients have demonstrated an oncologic benefit with neoadjuvant chemotherapy but at the expense of increased toxicities and decreased quality of life (92,93).
The role of neoadjuvant chemoradiation remains unclear and remains an active area of investigation (94-97). Additional studies will be needed to determine if the potential benefits are also applicable to the elderly population. Our investigation of the NCDB showed that among all the elderly patients who underwent radical gastrectomy, only 7.5% received neoadjuvant chemo/radiation therapy, whereas the proportion is much higher in other age groups (44.5% for patients <65 years and 33.6% for patients aged 65–79 years, P<0.001).
Adjuvant therapy for the elderly
For patients who did not receive neoadjuvant chemotherapy prior to undergoing resection, adjuvant chemotherapy or chemoradiation is recommended for patients with high risk of disease recurrence (98). The survival benefit of adjuvant chemotherapy for resected patients compared to surgery alone has been well documented in two randomized phase III trials from Asia. The CLASSIC trial reported an improved 3-year disease-free survival (DFS) of 74% in patients who underwent D2 gastrectomy followed by chemotherapy compared to 59% in patients who received surgery alone (99). In a subgroup analysis by age, patients ≥65 years receiving adjuvant chemotherapy also had improved 3-year DFS. In the ACTS GC trial, the 5-year DFS in patients who received adjuvant chemotherapy was 65% compared to 53% in the surgery-only group; 5-year OS was 72% and 61% in the adjuvant chemotherapy and surgery-only groups, respectively (100). However, in subgroup analysis by age, there was no difference in DFS for patients ≥60 years and no difference in OS for patients ≥70 years. To date, no randomized trials have been conducted to evaluate the benefit of adjuvant chemotherapy specifically in elderly patients who have undergone gastrectomy. Retrospective studies focusing on elderly patients have reported conflicting results and, without randomization, are difficult to interpret due to the potential effect of selection bias in which elderly patients received the addition of adjuvant chemotherapy (101-105).
Intergroup 0116 was the first randomized phase III trial conducted in Western patients to demonstrate the benefit of adjuvant chemoradiation after surgery compared to surgery alone (106). The initial study findings published in 2001 reported an improved median OS of 36 months in patients who received adjuvant chemoradiation compared to 27 months for those who had surgery only (106). The final update from this study cohort was published after more than ten years of follow-up and demonstrated a persistent benefit in both OS and DFS for patients who had received adjuvant chemoradiation (107). Yeh et al. used the Surveillance, Epidemiology, and End Results-Medicare database to review 1,519 patients ≥65 years of age who had undergone gastrectomy, of whom 42% received adjuvant chemoradiation (108). In this retrospective, population-based study, adjuvant chemoradiation demonstrated a survival benefit (HR 0.59, 95% CI: 0.50–0.67) over surgery alone, particularly for patients with stages II and III disease.
The benefit of adjuvant therapy continues to be supported in subsequent trials and reviews; however, the added benefit of adjuvant chemoradiation compared to adjuvant chemotherapy alone remains under debate (109-112). Future prospective clinical trials need to be conducted to study the potential benefit of adjuvant therapy in elderly patients and to determine the optimal treatment regimens to maximize disease control as well as maintain their quality of life.
Table 2 presents a review of chemotherapy, radiation, and chemoradiation trials and studies comprising gastric cancer patients of all ages. Table 3 summarizes trials and studies that focused specifically on the treatment of elderly patients.
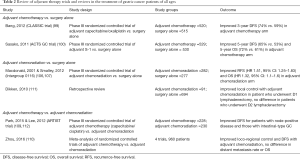
Full table

Full table
Treatment of metastatic disease for the elderly
In the setting of metastatic disease, combination therapy with platinum and fluoropyrimidines is recommended. In a multi-center phase III trial of 50 patients ≥70 years with metastatic cancer, double therapy with oxaliplatin and capecitabine improved both PFS and OS compared to monotherapy with capecitabine (PFS 7.1 vs. 2.6 months, OS 11.1 vs. 6.3 months) (113). This is comparable to the median OS times reported in phase III trials comprised of patients of all ages with metastatic gastric cancer (114-116). Sasaki et al. reported in a phase II trial that combination cisplatin and S-1, an oral fluoropyrimidine, was also safe and effective for elderly patients ≥76 years, with median PFS and OS of 7.8 and 12.3 months, respectively (117). In a sub-group analysis of patients ≥70 years who were enrolled in the Japanese randomized phase III G-SOX comparing S-1 and oxaliplatin with S-1 and cisplatin therapy, there were no differences in PFS or OS between the two combination regimens but elderly patients receiving S-1 and oxaliplatin experienced fewer toxicities (118). A retrospective review of 129 patients ≥65 years with metastatic or recurrent gastric cancer also showed that combination S-1 and oxaliplatin was well-tolerated in this older age group (119) (Table 4).

Full table
Summary statement
- Patient and tumor characteristics of elderly patients: Elderly patients tend to have more comorbidities and present with more advanced stage of disease. Histologically, elderly patients tend to have larger, multiple tumors of well-differentiated and intestinal-type histology.
- Surgery in elderly patients: Gastric cancer surgery, including total gastrectomy, is safe for physically-fit elderly patients. We recommend a minimally-invasive approach with less extensive lymph node dissection to minimize post-operative morbidity and mortality.
- Neoadjuvant therapy for the elderly: Based on clinical trials conducted among participants of all ages, patients with ≥T1b tumors are recommended to receive neoadjuvant chemotherapy with a platinum/fluoropyrimidine combination regimen. Among elderly patients, the oncologic benefit of neoadjuvant therapy must be balanced with the potentially increased toxicities and decreased quality of life.
- Adjuvant therapy for the elderly: Based on clinical trials conducted among participants of all ages, patients who did not receive neoadjuvant chemotherapy should receive adjuvant chemotherapy or chemoradiation. There is no strong evidence to support the additional benefit of adjuvant radiation therapy for patients who received adjuvant chemotherapy. To date, no randomized trials have been conducted to evaluate the benefit of adjuvant therapy specifically in elderly patients who have undergone gastrectomy.
- Treatment of metastatic disease for the elderly: Combination therapy with platinum and fluoropyrimidines is recommended in the setting of metastatic disease. This regimen has been shown to be safe and well-tolerated in elderly patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kitamura K, Yamaguchi T, Taniguchi H, et al. Clinicopathological characteristics of gastric cancer in the elderly. Br J Cancer 1996;73:798-802. [Crossref] [PubMed]
- Bittner R, Butters M, Ulrich M, et al. Total gastrectomy. Updated operative mortality and long-term survival with particular reference to patients older than 70 years of age. Ann Surg 1996;224:37-42. [Crossref] [PubMed]
- Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol 2006;12:354-62. [Crossref] [PubMed]
- Thakkar JP, McCarthy BJ, Villano JL. Age-specific cancer incidence rates increase through the oldest age groups. Am J Med Sci 2014;348:65-70. [Crossref] [PubMed]
- Li Y, Tan B, Fan L, et al. Clinicopathologic Characteristics of Elderly with Gastric Cancer, and the Risk Factors of Postoperative Complications. J Invest Surg 2017;30:394-400. [Crossref] [PubMed]
- Kuo CY, Chao Y, Li CP. Update on treatment of gastric cancer. J Chin Med Assoc 2014;77:345-53. [Crossref] [PubMed]
- Bray F, Jemal A, Grey N, et al. Global cancer transitions according to the Human Development Index (2008-2030): a population-based study. Lancet Oncol 2012;13:790-801. [Crossref] [PubMed]
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Balakrishnan M, George R, Sharma A, et al. Changing Trends in Stomach Cancer Throughout the World. Curr Gastroenterol Rep 2017;19:36. [Crossref] [PubMed]
- Yamamoto S. Stomach cancer incidence in the world. Jpn J Clin Oncol 2001;31:471. [PubMed]
- Sasako M, Inoue M, Lin JT, et al. Gastric Cancer Working Group report. Jpn J Clin Oncol 2010;40 Suppl 1:i28-37. [Crossref] [PubMed]
- Parsonnet J, Friedman GD, Vandersteen DP, et al. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med 1991;325:1127-31. [Crossref] [PubMed]
- Forman D, Newell DG, Fullerton F, et al. Association between infection with Helicobacter pylori and risk of gastric cancer: evidence from a prospective investigation. BMJ 1991;302:1302-5. [Crossref] [PubMed]
- Kobayashi T, Kikuchi S, Lin Y, et al. Trends in the incidence of gastric cancer in Japan and their associations with Helicobacter pylori infection and gastric mucosal atrophy. Gastric Cancer 2004;7:233-9. [Crossref] [PubMed]
- Take S, Mizuno M, Ishiki K, et al. Seventeen-year effects of eradicating Helicobacter pylori on the prevention of gastric cancer in patients with peptic ulcer; a prospective cohort study. J Gastroenterol 2015;50:638-44. [Crossref] [PubMed]
- Kim N, Park RY, Cho SI, et al. Helicobacter pylori infection and development of gastric cancer in Korea: long-term follow-up. J Clin Gastroenterol 2008;42:448-54. [Crossref] [PubMed]
- Inoue M. Changing epidemiology of Helicobacter pylori in Japan. Gastric Cancer 2017;20:3-7. [Crossref] [PubMed]
- Seta T, Takahashi Y, Noguchi Y, et al. Effectiveness of Helicobacter pylori eradication in the prevention of primary gastric cancer in healthy asymptomatic people: A systematic review and meta-analysis comparing risk ratio with risk difference. PLoS One 2017;12:e0183321. [Crossref] [PubMed]
- Hamashima C. Current issues and future perspectives of gastric cancer screening. World J Gastroenterol 2014;20:13767-74. [Crossref] [PubMed]
- Pasechnikov V, Chukov S, Fedorov E, et al. Gastric cancer: prevention, screening and early diagnosis. World J Gastroenterol 2014;20:13842-62. [Crossref] [PubMed]
- Kim H, Hwang Y, Sung H, et al. Effectiveness of Gastric Cancer Screening on Gastric Cancer Incidence and Mortality in a Community-Based Prospective Cohort. Cancer Res Treat 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Jun JK, Choi KS, Lee HY, et al. Effectiveness of the Korean National Cancer Screening Program in Reducing Gastric Cancer Mortality. Gastroenterology 2017;152:1319-28.e7. [Crossref] [PubMed]
- Pourfarzi F, Whelan A, Kaldor J, et al. The role of diet and other environmental factors in the causation of gastric cancer in Iran--a population based study. Int J Cancer 2009;125:1953-60. [Crossref] [PubMed]
- Moy KA, Fan Y, Wang R, et al. Alcohol and tobacco use in relation to gastric cancer: a prospective study of men in Shanghai, China. Cancer Epidemiol Biomarkers Prev 2010;19:2287-97. [Crossref] [PubMed]
- Sjodahl K, Lu Y, Nilsen TI, et al. Smoking and alcohol drinking in relation to risk of gastric cancer: a population-based, prospective cohort study. Int J Cancer 2007;120:128-32. [Crossref] [PubMed]
- Lin SH, Li YH, Leung K, et al. Salt processed food and gastric cancer in a Chinese population. Asian Pac J Cancer Prev 2014;15:5293-8. [Crossref] [PubMed]
- Yang WG, Chen CB, Wang ZX, et al. A case-control study on the relationship between salt intake and salty taste and risk of gastric cancer. World J Gastroenterol 2011;17:2049-53. [Crossref] [PubMed]
- Fang X, Wei J, He X, et al. Landscape of dietary factors associated with risk of gastric cancer: A systematic review and dose-response meta-analysis of prospective cohort studies. Eur J Cancer 2015;51:2820-32. [Crossref] [PubMed]
- Stojanovic J, Giraldi L, Arzani D, et al. Adherence to Mediterranean diet and risk of gastric cancer: results of a case-control study in Italy. Eur J Cancer Prev 2017;26:491-6. [Crossref] [PubMed]
- Wang T, Cai H, Sasazuki S, et al. Fruit and vegetable consumption, Helicobacter pylori antibodies, and gastric cancer risk: A pooled analysis of prospective studies in China, Japan, and Korea. Int J Cancer 2017;140:591-9. [Crossref] [PubMed]
- Ramesh HS, Pope D, Gennari R, et al. Optimising surgical management of elderly cancer patients. World J Surg Oncol 2005;3:17. [Crossref] [PubMed]
- Why Population Aging Matters: A Global Perspective. 2007. Accessed September 5 2017. Available online: https://www.nia.nih.gov/sites/default/files/2017-06/WPAM.pdf
- Eguchi T, Fujii M, Takayama T. Mortality for gastric cancer in elderly patients. J Surg Oncol 2003;84:132-6. [Crossref] [PubMed]
- Wu CW, Lo SS, Shen KH, et al. Surgical mortality, survival, and quality of life after resection for gastric cancer in the elderly. World J Surg 2000;24:465-72. [Crossref] [PubMed]
- Hsu JT, Liu MS, Wang F, et al. Standard radical gastrectomy in octogenarians and nonagenarians with gastric cancer: are short-term surgical results and long-term survival substantial? J Gastrointest Surg 2012;16:728-37. [Crossref] [PubMed]
- Park HJ, Ahn JY, Jung HY, et al. Clinical Characteristics and Outcomes of Gastric Cancer Patients Aged over 80 Years: A Retrospective Case-Control Study. PLoS One 2016;11:e0167615. [Crossref] [PubMed]
- Kim JH, Chin HM, Jun KH. Surgical outcomes and survival after gastrectomy in octogenarians with gastric cancer. J Surg Res 2015;198:80-6. [Crossref] [PubMed]
- Arai T, Esaki Y, Inoshita N, et al. Pathologic characteristics of gastric cancer in the elderly: a retrospective study of 994 surgical patients. Gastric Cancer 2004;7:154-9. [Crossref] [PubMed]
- Medina-Franco H, Heslin MJ, Cortes-Gonzalez R. Clinicopathological characteristics of gastric carcinoma in young and elderly patients: a comparative study. Ann Surg Oncol 2000;7:515-9. [Crossref] [PubMed]
- Kunisaki C, Akiyama H, Nomura M, et al. Comparison of surgical outcomes of gastric cancer in elderly and middle-aged patients. Am J Surg 2006;191:216-24. [Crossref] [PubMed]
- Kim DY, Joo JK, Ryu SY, et al. Clinicopathologic characteristics of gastric carcinoma in elderly patients: a comparison with young patients. World J Gastroenterol 2005;11:22-6. [Crossref] [PubMed]
- Otsuji E, Fujiyama J, Takagi T, et al. Results of total gastrectomy with extended lymphadenectomy for gastric cancer in elderly patients. J Surg Oncol 2005;91:232-6. [Crossref] [PubMed]
- Brenkman HJ, Goense L, Brosens LA, et al. A High Lymph Node Yield is Associated with Prolonged Survival in Elderly Patients Undergoing Curative Gastrectomy for Cancer: A Dutch Population-Based Cohort Study. Ann Surg Oncol 2017;24:2213-23. [Crossref] [PubMed]
- Kolodziejczyk P, Kulig J, Popiela T, et al. Outcome of gastric cancer surgery in elderly patients. Hepatogastroenterology 2005;52:1911-5. [PubMed]
- Cho GS, Kim W, Kim HH, et al. Multicentre study of the safety of laparoscopic subtotal gastrectomy for gastric cancer in the elderly. Br J Surg 2009;96:1437-42. [Crossref] [PubMed]
- Liang YX, Deng JY, Guo HH, et al. Characteristics and prognosis of gastric cancer in patients aged >/= 70 years. World J Gastroenterol 2013;19:6568-78. [Crossref] [PubMed]
- Saif MW, Makrilia N, Zalonis A, et al. Gastric cancer in the elderly: an overview. Eur J Surg Oncol 2010;36:709-17. [Crossref] [PubMed]
- Lim JH, Lee DH, Shin CM, et al. Clinicopathological features and surgical safety of gastric cancer in elderly patients. J Korean Med Sci 2014;29:1639-45. [Crossref] [PubMed]
- Saito H, Osaki T, Murakami D, et al. Effect of age on prognosis in patients with gastric cancer. ANZ J Surg 2006;76:458-61. [Crossref] [PubMed]
- Maehara Y, Emi Y, Tomisaki S, et al. Age-related characteristics of gastric carcinoma in young and elderly patients. Cancer 1996;77:1774-80. [Crossref] [PubMed]
- Edge S, Byrd DR, Compton CC, et al. AJCC Cancer Staging Manual. 7th ed. New York: Springer, 2010.
- Smyth EC, Verheij M, Allum W, et al. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v38-49. [Crossref] [PubMed]
- Fujiwara Y, Fukuda S, Tsujie M, et al. Effects of age on survival and morbidity in gastric cancer patients undergoing gastrectomy. World J Gastrointest Oncol 2017;9:257-62. [Crossref] [PubMed]
- Endo S, Shimizu Y, Ikenaga M, et al. Survival benefit of gastrectomy for gastric cancer in patients >/=85 years old: A retrospective propensity score-matched analysis. Surgery 2017;161:984-94. [Crossref] [PubMed]
- Yamada H, Shinohara T, Takeshita M, et al. Postoperative complications in the oldest old gastric cancer patients. Int J Surg 2013;11:467-71. [Crossref] [PubMed]
- Kiuchi J, Komatsu S, Ichikawa D, et al. Putative risk factors for postoperative pneumonia which affects poor prognosis in patients with gastric cancer. Int J Clin Oncol 2016;21:920-6. [Crossref] [PubMed]
- Inokuchi M, Kojima K, Kato K, et al. Risk factors for post-operative pulmonary complications after gastrectomy for gastric cancer. Surg Infect (Larchmt) 2014;15:314-21. [Crossref] [PubMed]
- Liu CA, Huang KH, Chen MH, et al. Comparison of the surgical outcomes of minimally invasive and open surgery for octogenarian and older compared to younger gastric cancer patients: a retrospective cohort study. BMC Surg 2017;17:68. [Crossref] [PubMed]
- de Miguel-Diez J, Lopez-de-Andres A, Hernandez-Barrera V, et al. Decreasing incidence and mortality among hospitalized patients suffering a ventilator-associated pneumonia: Analysis of the Spanish national hospital discharge database from 2010 to 2014. Medicine (Baltimore) 2017;96:e7625. [Crossref] [PubMed]
- Rello J, Ollendorf DA, Oster G, et al. Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. Chest 2002;122:2115-21. [Crossref] [PubMed]
- Angele MK, Ayala A, Monfils BA, et al. Testosterone and/or low estradiol: normally required but harmful immunologically for males after trauma-hemorrhage. J Trauma 1998;44:78-85. [Crossref] [PubMed]
- Reade MC, Yende S, D'Angelo G, et al. Differences in immune response may explain lower survival among older men with pneumonia. Crit Care Med 2009;37:1655-62. [Crossref] [PubMed]
- Knoferl MW, Angele MK, Diodato MD, et al. Female sex hormones regulate macrophage function after trauma-hemorrhage and prevent increased death rate from subsequent sepsis. Ann Surg 2002;235:105-12. [Crossref] [PubMed]
- Yang CK, Teng A, Lee DY, et al. Pulmonary complications after major abdominal surgery: National Surgical Quality Improvement Program analysis. J Surg Res 2015;198:441-9. [Crossref] [PubMed]
- Hasukic S, Mesic D, Dizdarevic E, et al. Pulmonary function after laparoscopic and open cholecystectomy. Surg Endosc 2002;16:163-5. [Crossref] [PubMed]
- Fernandez-Bustamante A, Frendl G, Sprung J, et al. Postoperative Pulmonary Complications, Early Mortality, and Hospital Stay Following Noncardiothoracic Surgery: A Multicenter Study by the Perioperative Research Network Investigators. JAMA Surg 2017;152:157-66. [Crossref] [PubMed]
- Colucci DB, Fiore JF Jr, Paisani DM, et al. Cough impairment and risk of postoperative pulmonary complications after open upper abdominal surgery. Respir Care 2015;60:673-8. [Crossref] [PubMed]
- Lai-Fook SJ, Hyatt RE. Effects of age on elastic moduli of human lungs. J Appl Physiol (1985) 2000;89:163-8. [PubMed]
- Janssens JP. Aging of the respiratory system: impact on pulmonary function tests and adaptation to exertion. Clin Chest Med 2005;26:469-84. vi-vii. [Crossref] [PubMed]
- Persiani R, Antonacci V, Biondi A, et al. Determinants of surgical morbidity in gastric cancer treatment. J Am Coll Surg 2008;207:13-9. [Crossref] [PubMed]
- Lee KG, Lee HJ, Yang JY, et al. Risk factors associated with complication following gastrectomy for gastric cancer: retrospective analysis of prospectively collected data based on the Clavien-Dindo system. J Gastrointest Surg 2014;18:1269-77. [Crossref] [PubMed]
- Gretschel S, Estevez-Schwarz L, Hunerbein M, et al. Gastric cancer surgery in elderly patients. World J Surg 2006;30:1468-74. [Crossref] [PubMed]
- Kwon IG, Cho I, Guner A, et al. Minimally invasive surgery as a treatment option for gastric cancer in the elderly: comparison with open surgery for patients 80 years and older. Surg Endosc 2015;29:2321-30. [Crossref] [PubMed]
- Inokuchi M, Tanioka T, Nakagawa M, et al. Laparoscopic Distal Gastrectomy is Feasible in Very Elderly Patients as Compared with Open Distal Gastrectomy. J Invest Surg 2017.1-7. [Crossref] [PubMed]
- Yasuda K, Sonoda K, Shiroshita H, et al. Laparoscopically assisted distal gastrectomy for early gastric cancer in the elderly. Br J Surg 2004;91:1061-5. [Crossref] [PubMed]
- Okumura N, Son T, Kim YM, et al. Robotic gastrectomy for elderly gastric cancer patients: comparisons with robotic gastrectomy in younger patients and laparoscopic gastrectomy in the elderly. Gastric Cancer 2016;19:1125-34. [Crossref] [PubMed]
- Kitano S, Shiraishi N, Fujii K, et al. A randomized controlled trial comparing open vs laparoscopy-assisted distal gastrectomy for the treatment of early gastric cancer: an interim report. Surgery 2002;131:S306-11. [Crossref] [PubMed]
- Suzuki S, Nakamura T, Imanishi T, et al. Carbon dioxide pneumoperitoneum led to no severe morbidities for the elderly during laparoscopic-assisted distal gastrectomy. Ann Surg Oncol 2015;22:1548-54. [Crossref] [PubMed]
- Yamada H, Kojima K, Inokuchi M, et al. Laparoscopy-assisted gastrectomy in patients older than 80. J Surg Res 2010;161:259-63. [Crossref] [PubMed]
- Tokunaga M, Hiki N, Fukunaga T, et al. Does age matter in the indication for laparoscopy-assisted gastrectomy? J Gastrointest Surg 2008;12:1502-7. [Crossref] [PubMed]
- Kunisaki C, Makino H, Takagawa R, et al. Efficacy of laparoscopy-assisted distal gastrectomy for gastric cancer in the elderly. Surg Endosc 2009;23:377-83. [Crossref] [PubMed]
- Kim KH, Kim MC, Jung GJ. Is the rate of postoperative complications following laparoscopy-assisted gastrectomy higher in elderly patients than in younger patients? World J Surg Oncol 2014;12:97. [Crossref] [PubMed]
- Wang JF, Zhang SZ, Zhang NY, et al. Laparoscopic gastrectomy versus open gastrectomy for elderly patients with gastric cancer: a systematic review and meta-analysis. World J Surg Oncol 2016;14:90. [Crossref] [PubMed]
- Hartgrink HH, van de Velde CJ, Putter H, et al. Extended lymph node dissection for gastric cancer: who may benefit? Final results of the randomized Dutch gastric cancer group trial. J Clin Oncol 2004;22:2069-77. [Crossref] [PubMed]
- Rausei S, Ruspi L, Rosa F, et al. Extended lymphadenectomy in elderly and/or highly co-morbid gastric cancer patients: A retrospective multicenter study. Eur J Surg Oncol 2016;42:1881-9. [Crossref] [PubMed]
- Passot G, Vaudoyer D, Messager M, et al. Is Extended Lymphadenectomy Needed for Elderly Patients With Gastric Adenocarcinoma? Ann Surg Oncol 2016;23:2391-7. [Crossref] [PubMed]
- Eguchi T, Takahashi Y, Ikarashi M, et al. Is extended lymph node dissection necessary for gastric cancer in elderly patients? Eur J Surg 2000;166:949-53. [Crossref] [PubMed]
- Takeshita H, Ichikawa D, Komatsu S, et al. Surgical outcomes of gastrectomy for elderly patients with gastric cancer. World J Surg 2013;37:2891-8. [Crossref] [PubMed]
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 2011;29:1715-21. [Crossref] [PubMed]
- Brenner B, Shah MA, Karpeh MS, et al. A phase II trial of neoadjuvant cisplatin-fluorouracil followed by postoperative intraperitoneal floxuridine-leucovorin in patients with locally advanced gastric cancer. Ann Oncol 2006;17:1404-11. [Crossref] [PubMed]
- Al-Batran SE, Pauligk C, Homann N, et al. The feasibility of triple-drug chemotherapy combination in older adult patients with oesophagogastric cancer: a randomised trial of the Arbeitsgemeinschaft Internistische Onkologie (FLOT65+). Eur J Cancer 2013;49:835-42. [Crossref] [PubMed]
- Lorenzen S, Pauligk C, Homann N, et al. Feasibility of perioperative chemotherapy with infusional 5-FU, leucovorin, and oxaliplatin with (FLOT) or without (FLO) docetaxel in elderly patients with locally advanced esophagogastric cancer. Br J Cancer 2013;108:519-26. [Crossref] [PubMed]
- Stahl M, Walz MK, Stuschke M, et al. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol 2009;27:851-6. [Crossref] [PubMed]
- Walsh TN, Noonan N, Hollywood D, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med 1996;335:462-7. [Crossref] [PubMed]
- Leong T, Smithers BM, Haustermans K, et al. TOPGEAR: A Randomized, Phase III Trial of Perioperative ECF Chemotherapy with or Without Preoperative Chemoradiation for Resectable Gastric Cancer: Interim Results from an International, Intergroup Trial of the AGITG, TROG, EORTC and CCTG. Ann Surg Oncol 2017;24:2252-8. [Crossref] [PubMed]
- Shapiro J, van Lanschot JJ, Hulshof M, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. [Crossref] [PubMed]
- Network NCC. NCCN Guidelines Gastric Cancer. Version 4.2017. Accessed October 6 2017. Available online: https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf
- Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet 2012;379:315-21. [Crossref] [PubMed]
- Sasako M, Sakuramoto S, Katai H, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol 2011;29:4387-93. [Crossref] [PubMed]
- Jeong JW, Kwon IG, Son YG, et al. Could Adjuvant Chemotherapy after Surgery Benefit Elderly Patients with Advanced Gastric Cancer? J Gastric Cancer 2016;16:260-5. [Crossref] [PubMed]
- Jo JC, Baek JH, Koh SJ, et al. Adjuvant chemotherapy for elderly patients (aged 70 or older) with gastric cancer after a gastrectomy with D2 dissection: A single center experience in Korea. Asia Pac J Clin Oncol 2015;11:282-7. [Crossref] [PubMed]
- Jin Y, Qiu MZ, Wang DS, et al. Adjuvant chemotherapy for elderly patients with gastric cancer after D2 gastrectomy. PLoS One 2013;8:e53149. [Crossref] [PubMed]
- Hanazaki K, Mochizuki Y, Igarashi J, et al. Postoperative chemotherapy in elderly patients with advanced gastric cancer. Hepatogastroenterology 2000;47:1761-4. [PubMed]
- Maehara Y, Yamamoto M, Endo K, et al. Postoperative chemotherapy for gastric cancer in the elderly. Chemotherapy 1994;40:279-86. [Crossref] [PubMed]
- Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 2001;345:725-30. [Crossref] [PubMed]
- Smalley SR, Benedetti JK, Haller DG, et al. Updated analysis of SWOG-directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol 2012;30:2327-33. [Crossref] [PubMed]
- Yeh JM, Tramontano AC, Hur C, et al. Comparative effectiveness of adjuvant chemoradiotherapy after gastrectomy among older patients with gastric adenocarcinoma: a SEER-Medicare study. Gastric Cancer 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Park SH, Sohn TS, Lee J, et al. Phase III Trial to Compare Adjuvant Chemotherapy With Capecitabine and Cisplatin Versus Concurrent Chemoradiotherapy in Gastric Cancer: Final Report of the Adjuvant Chemoradiotherapy in Stomach Tumors Trial, Including Survival and Subset Analyses. J Clin Oncol 2015;33:3130-6. [Crossref] [PubMed]
- Zhou ML, Kang M, Li GC, et al. Postoperative chemoradiotherapy versus chemotherapy for R0 resected gastric cancer with D2 lymph node dissection: an up-to-date meta-analysis. World J Surg Oncol 2016;14:209. [Crossref] [PubMed]
- Dikken JL, Jansen EP, Cats A, et al. Impact of the extent of surgery and postoperative chemoradiotherapy on recurrence patterns in gastric cancer. J Clin Oncol 2010;28:2430-6. [Crossref] [PubMed]
- Lee J, Lim DH, Kim S, et al. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol 2012;30:268-73. [Crossref] [PubMed]
- Hwang IG, Ji JH, Kang JH, et al. A multi-center, open-label, randomized phase III trial of first-line chemotherapy with capecitabine monotherapy versus capecitabine plus oxaliplatin in elderly patients with advanced gastric cancer. J Geriatr Oncol 2017;8:170-5. [Crossref] [PubMed]
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [Crossref] [PubMed]
- Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2008;358:36-46. [Crossref] [PubMed]
- Ohtsu A, Shah MA, Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol 2011;29:3968-76. [Crossref] [PubMed]
- Sasaki Y, Iwasa S, Okazaki S, et al. A phase II study of combination therapy with oral S-1 and cisplatin in elderly patients with advanced gastric cancer. Gastric Cancer 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Bando H, Yamada Y, Tanabe S, et al. Efficacy and safety of S-1 and oxaliplatin combination therapy in elderly patients with advanced gastric cancer. Gastric Cancer 2016;19:919-26. [Crossref] [PubMed]
- Zhong DT, Wu RP, Wang XL, et al. Combination Chemotherapy with S-1 and Oxaliplatin (SOX) as First-Line Treatment in Elderly Patients with Advanced Gastric Cancer. Pathol Oncol Res 2015;21:867-73. [Crossref] [PubMed]
Cite this article as: Pak LM, Wang J. The appropriate treatment for elderly gastric cancer patients. Art Surg 2017;1:4.


