
Current and developing immunotherapy in gastric adenocarcinoma
Introduction
Gastric cancer (GC) falls into a category of tumors that exhibit a robust anti-tumor immune response. GC ranks fifth in regards to the neoantigen repertoire in human cancer, which signifies a consistent response to cancer immunotherapy. According to The Cancer Genome Atlas (TCGA) molecular classification of GC, Epstein-Barr virus (EBV) positive GC has shown evidence of PD-L1/2 overexpression. GC with microsatellite instability (MSI) has demonstrated elevated mutation rates and rates of hypermethylation, indicating further benefit from immunotherapy (1,2). The immune response to GC evolves during its progression with various stages exhibiting particular immune signatures (3). For example, certain sets of T-cell subsets have been shown to be prognostic in GC. There is differential prognostic predictive value based on the predominant type of infiltration or immune signature (4). As solid tumors have differential immune cell infiltration, they are also variable as to their ability to mount an immune response to immunotherapy, exhibited by the differential responses between cancer types. As certain “cold” tumors such as pancreatic and prostate cancer fail to have remarkable responses to immune based therapy, GC has been shown to have clinical responses in various treatment settings. Given its immunogenicity and promising results in preclinical studies, there is a growing interest in harboring the immune response to promote an anti-tumor antigen-specific immune response.
Various strategies are being employed to enhance the immune response and augment its anti-tumor effects. In addition, much attention has been placed on blocking inhibitory costimulatory receptors that have been shown to blunt the anti-tumor immune response. Therapies in GC which act to provide and enhance a tumor-specific adaptive immune response largely include adoptive cell therapy (ACT) and therapeutic vaccines. With the advent and avid use of checkpoint blockade in other cancer types, there has been strong interest in employing this strategy in GC. Furthermore, the use of combinatorial therapy with standard therapies or targeted therapies along with immunotherapy is currently being investigated in multiple ongoing clinical trials with exciting results on the horizon. In this review, we highlight the major types of immunotherapy that are currently being investigated clinically and current ongoing clinical trials for each therapy subtype in GC.
ACT
ACT is the process by which immune cells are harvested from a patient with cancer and expanded ex vivo in a tumor-specific manner, usually targeted to known tumor-associated antigens (TAAs). Once expanded, these immune cells are given back to the patient as a therapy. There are variety ACT strategies, each of which harness various cells involved in the immune response to include, dendritic cells (DCs), natural-killer cells (NK cells), both of which are derived from the innate immune response, and T-lymphocytes (T cells) derived from the adaptive immune response. Here we will discuss two examples of ACT currently being investigated in GC. In addition, Table 1 lists clinical trials involving adoptive cell therapies in GC.
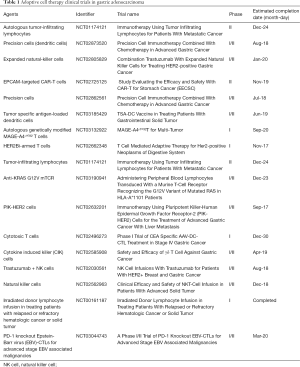
Full table
Early investigations with ACT using T cells in GC were harvested from patient-derived tumor tissue and expanded prior to infusion into the patient. Kono et al. evaluated tumor-associated lymphocytes in combination with chemotherapy in advanced stage GC (5). T cells were harvested from suspension prepared from malignant ascites or pleural effusions as well as lymph node metastases in GC patients. Cells were generated by culturing and expanding an enriched T-cell population. Repetitive stimulation with the autologous tumor was used during expansion to derive an antigen specific T-cell population. Twenty-two patients were evaluated in each arm of their study. Median survival was found to be 11.3 months in the combination chemotherapy plus ACT group versus 8.3 months in the chemotherapy only group (P<0.05). Additionally, treatment with adoptive T cells were found to independently predict survival in the cohort of patients in their multivariate analysis (5).
Another adoptive strategy that has been employed in GC is the use of activated tumor-specific lymphocytes combining various lymphocyte subtypes, all of which are CD3+ T cells, including T-helper cells (CD4+), cytotoxic T cells (CTLs, CD8+), as well as NK cells. These pooled lymphocytes are termed expanded activated autologous lymphocytes (EAALs) (6). Zhang et al. retrospectively analyzed their experience of GC patients treated with EAALs (7). Lymphocytes were harvested by isolating peripheral blood mononuclear cells from patients which were subsequently activated and proliferated using IL-2. These cells were infused back into patients. Retrospective analysis of 42 patients with various clinical stages of GC who had undergone EAAL treatment in addition to chemotherapy, radiotherapy, or surgery were compared to 42 similar GC patients from the same institution. Patients treated with EAAL had better median OS (27.0 vs. 13.9 months, P=0.028) compared to patients that were not. In addition, EAAL treatment was found to be an independent predictor of OS on multivariate analysis (7). Although the two patient cohorts were similar, the design of the study was not prospectively randomized and not ideal to compare the two groups. Therefore, conclusions in regard to its value as a sustainable immunotherapeutic strategy in GC are limited. Nevertheless, this study was the first to evaluate EAALs in GC patients and demonstrates its potential for utility. Further investigation is warranted in a prospectively randomized fashion.
Cytokine induced killer (CIK) cells are yet another example of adoptive T-cell therapy. In this strategy, ex vivo cultured T cells derived from GC patients are expanded and stimulated with interferon-gamma, anti-CD3 antibody, IL-1 and IL-2 leading to their distinctive phenotype. CIKs include a mixed T-cell and NK cell-like phenotype and, together, possess a combination of an major histocompatibility complex (MHC)-restricted and MHC-unrestricted anti-tumor effect. The NK cells are responsible MHC-unrestricted activity which is thought to broaden the anti-tumor effect by its ability to activate without MHC binding (8). Conversely, this type of interaction is required by T cells for activation of a TAA-specific effect. Jiang and colleagues evaluated 52 stage IV GC patients and compared chemotherapy plus CIK therapy versus and chemotherapy alone (9). Survival was improved in the group treated with chemotherapy plus CIK cells compared to the group treated with chemotherapy alone within 2 years of treatment. However, there was no difference between the two groups after 2 years with survival curves overlapping after this time period (9). This represents early treatment effect with likely resistance developing in patients who survived longer than 2 years. Identifying potential mechanisms of such resistance are necessary for the clinical utility of CIK cells. Mechanistically understanding resistance to various types immunotherapy by cancer cells represents a growing field of study which continues to clarify important immunological questions about the tumor microenvironment. A meta-analysis including 318 GC patients receiving CIK cell therapy and 369 patients receiving conventional therapy demonstrated an increased 5-year survival rate among patients receiving CIK cell therapy (10).
Although the available data for the support of adoptive therapy in GC provide strong evidence for its continued investigation, there is no convincing evidence to support its current use as standard therapy, even in combination with chemotherapy, in patients with GC. Newer ACTs under investigation in GC include chimeric antigen receptors T cells (CARs), which are T cells engineered to express receptors specific to a defined TAA. CARs have demonstrated positive results in other cancer types and are currently being evaluated in clinical trials in GC with CARs specific for various known GC-associated antigens including HER2, CEA, MUC1 and EpCAM (11). CARs also have an advantage of being MHC-unrestricted which allows broad clinical use in patients with varying phenotypes.
In addition, several publications showed a surprising clinical response using adoptive transfer of T cells with T-cell receptors (TCRs) that have specificity for undruggable neoantigens, such as KRAS and ERBB2IP (12-14). T-cell therapy recognizing oncogenic virus neoantigens have also shown a significant immune response in vitro and in vivo, especially after PD-1 has been knocked out (15). This personalized model of neoantigen specific T-cell therapy is time-consuming because of the de novo peptide synthesis. However, it opens a promising window for the treatment of a wide variety of cancers.
GC vaccines
The use of cancer vaccines as an immunotherapy are designed to elicit an enhanced antigen-specific T-cell response. A variety of vaccines exist, including vaccines to known TAAs which are derived from an antigen of interest and contain the portion of the antigen that is normally identified by a TCR during its interaction with an MHC-peptide complex, a necessary step for T-cell activation. Alternatively, DC vaccines are used as another method to enhance antigen presenting capability against the specific TAA of interest in order to generate an enhanced antigen-specific T-cell response.
In GC, vaccines targeting assorted GC-associated antigens are being investigated. Table 2 lists registered vaccine trials in GC. Masuzawa and colleagues evaluated the safety and efficacy of vaccination with vascular endothelial growth factor (VEGF) peptides, specifically HLA-A2 restricted VEGF1-1084 and VEGF2-169 in advanced or recurrent GC (16). Out of the 22 patients, all which had had received at least one cycle of chemotherapy, 12 and 10 patients demonstrated partial response and stable disease, respectively. Eighteen of 122 patients demonstrated CTL response to the VEGF2-169 vaccine, and demonstrated better OS compared to patients without response (P=0.028). Additionally, there was an acceptable safety profile demonstrated in the study (16).
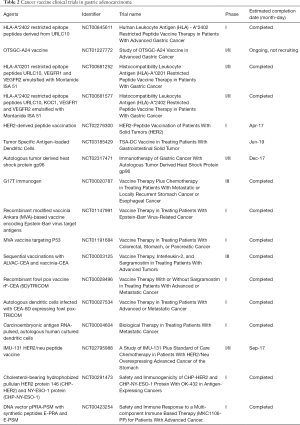
Full table
The concept of DC-based cancer vaccination is derived by its role as the primary antigen presenting cell (APC). T cells recognize antigens presented by DCs via detection by the TCR via an interaction to the MHC-peptide complex, leading to activation and proliferation of antigen specific T-cell. In this way, DCs can be incubated with TAAs of interest ex vivo and then can be infused into patients for native T cells to recognize. In a study evaluating DC-based vaccination by pulsing DCs with HER-2 derived peptide (p369) and administering vaccine to 9 HER-2 overexpressing GC patients, peptide-specific recognition by CTLs were demonstrated in 6 patients. One of the 6 patients underwent a partial response, and another patient demonstrated a stabilization of disease for 3 months. Importantly, none of the patients demonstrated adverse effects of the treatment demonstrating its safety (17). This data along with results from other investigators demonstrating antigen specific responses establish preliminary evidence for the support of the future investigation in a larger subset of GC patients. The limitation of DC-based vaccines are that CTLs can acquire activity that facilitates the early removal of DCs; this limits the ability of DCs to function in the continual propagation of an antigen specific CTL immune response (18). A Phase I clinical trial of HLA-A*2402-restricted LY6K-derived peptide LY6K-177 was conducted to evaluate the cytotoxicity in unresectable GC patients (19). Twelve gastrointestinal cancer patients were immunized with DCs loaded HLA-A2-restricted peptide MAGE-A3-271 and HLA-A24-restricted peptide MAGE-A3-195. Peptide-specific CTL responses were detectable in 50% (4/8) of patients without toxic side effects after vaccination (20).
Two recent promising studies reported a long term of disease free survival in melanoma patients after treatment of neoantigen-based tumor vaccines (21,22). This safe and personalized neoantigen vaccine can target highly heterogeneous tumors and minimize the chances of tumor escape by loss of antigen recognition, this meriting the value of validating these vaccines in GC patients validated in GC patients.
Checkpoint blockade
Perhaps the most exciting and promising strategy in the field of immunotherapy in the last decade has been the use of checkpoint blockade therapy. The rationale behind checkpoint blockade is to block inhibitory signals which normally deactivate T-cell effector functions (23). There are various checkpoint molecules that are being investigated. The most widely implemented clinically are CTLA-4 and PD-1. Their interaction with their highest affinity ligands, B7 and PD-L1, respectively, lead to downregulation of T-cell effector function (24,25). In its simplest explanation, this interaction normally prevents immune over-activation and subsequent collateral tissue damage in physiologic conditions (26). However, cancer cells including GC have been shown to overexpress ligands for both CTLA-4 and PD-1 (27,28). This mechanism of immune evasion by the cancer cell is the target of checkpoint blockade therapy.
The enthusiasm to harness these molecules as immune therapy has increased over the past decade and reached a peak in 2011 with the FDA approval of the anti-CTLA-4 antibody, ipilimumab which was approved for the treatment of unresectable stage III/IV melanoma (23,29). Since that period, there has been an outburst of investigators searching for ways to harness the aid of checkpoint blockade monoclonal antibodies to CTLA-4, and to PD-1 and its ligand PD-L1, in addition to other checkpoint molecules, in practically all cancer types. As in other disease sites, investigators have evaluated its use alone and in the combinatorial setting along with chemotherapy, radiotherapy, targeted therapies, as well as other immunotherapies. With the superior toxicity profile of anti-PD-1/PD-L1 therapies in comparison to anti-CTLA-4 therapy, many of the current clinical trials are focusing efforts on this interaction. With regards to GC, we will review important studies which have evaluated blockade each of these checkpoint molecules. We will also touch on strategies which may be employed to enhance combination therapies involving checkpoint blockade therapy.
CTLA-4 is expressed on CTLs, T-helper cells, as well as T regulatory cells (Tregs). Its interaction with its highest affinity ligand, B-7 leads to downregulation of T-cell effector function. This interaction is present in GC and has been studied in numerous clinical trials. Tremelimumab is a humanized monoclonal antibody that blocks the CTLA-4 interaction with its ligands. Its efficacy was evaluated in GC in a phase II trial (30). This was evaluated as second line treatment in metastatic GC and esophageal adenocarcinoma. Eighteen patients were treated with tremelimumab. Only 4 patients had stable disease at least 25.4 months from treatment with one achieving a durable response rate at least 32.7 months from treatment. Selected tumor markers (CA 19-9 and CEA) were evaluated as a method for assessing response in these patients. Overall survival (OS) for the entire group of advanced stage patients was 4.83 months. Median OS for patients with stable disease or those with decreasing levels of serum markers was 12.2 vs. 4.6 months (P=0.03). These results suggest that the anti-CTLA-4 therapy may have survival benefit but only in the small proportion of patients with objective response to therapy. Identifying mechanisms of enhancing response as well as reliable biomarkers to assess response to therapy may be beneficial in increasing efficacy and identifying the appropriate subset of patients who would benefit from therapy. To that end, combination therapy with CTLA-4 is being evaluated in multiple clinical trials.
PD-1 is also a co-inhibitory molecule expressed on T cells. Its ligands are PD-L1, which is the predominant ligand, and PD-L2. PD-L1 is expressed on many tumors and as well as normal tissues, in addition to immune cells. This interaction between PD-1 and PD-L1 also serves to suppress T-cell effector function in numerous cancer types including GC (28,31). In recent years, the FDA has approved use of various checkpoint inhibitor antibodies to both PD-1 (pembrolizumab, nivolumab) and PD-L1 (avelumab). Recently, pembrolizumab has been FDA approved for the use in metastatic GC and gastroesophageal junction cancers whose tumors express PD-L1. This expedited approval by the FDA was given based off of evidence from the KEYNOTE-059 trial which enrolled 259 patients who progressed on at least two prior systemic treatments for advanced disease. One hundred and forty-nine patients expressed PD-L1 within their tumors. These patients had an objective response rate (ORR) of 13.3%. Of the 19 responding patients, 11 had responses of 6 months or longer and 5 had responses of 12 months or longer (NCT02335411) (32). These data show that patients with PD-L1 expression in particular demonstrated acceptable ORRs. PD-L1 expression as a biomarker of response to anti-PD-L1 and anti-PD-1 therapy is debatable and continues to be investigated. While its overexpression may be beneficial, it does not seem to be a necessary prerequisite for response. In advanced melanoma for example, nivolumab has shown significant survival benefit response in PD-L1 negative tumors with a hazard ratio for death of 0.48 (95% CI, 0.32–0.71) in previously untreated patients (33). These results may be attributed to the fact that PD-L1 is not only expressed on tumor cells, but also in normal tissues.
Table 3 lists clinical trials involving anti-CTLA-4, PD-1, and PD-L1 checkpoint blockade therapies in GC.
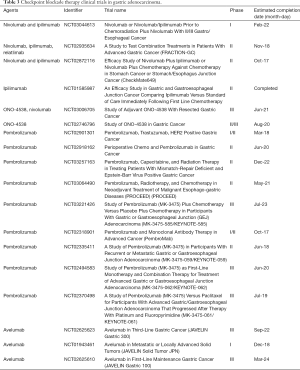
Full table
Rationale for chemotherapy, radiotherapy and targeted-therapy in combination with immunotherapy
The premise behind combining existing therapies with immunotherapy is the introduction of newly released tumor antigen for APC uptake and subsequent presentation to the immune system allowing for an enhanced tumor-antigen specific response. Furthermore, differing therapies may also result in a distinctive peptide profile which can lead to an augmented immune response depending on the potency of response elicited by the immunodominant peptides. In this section we discuss the potential and rationale for the use of various therapies in combination with immune based therapy.
The use of chemotherapy in GC may be able to modulate immune cell function. This effect may be in favor of the anti-tumoral immune response as cell death resulting from chemotherapeutic effect can lead to enhanced antigen delivery to APCs. In addition, chemotherapy may also be responsible for decreasing the load of immune suppressive cells such as Tregs (34). As an example, platinum-based chemotherapy in combination with immune checkpoint inhibitors have led to enhanced T-cell infiltration in lung cancer (35). In a phase I/II study in small-cell lung cancer patients, treatment with anti-PD-1 therapy plus dual platinum-based chemotherapy resulted in an ORR which was favorable in comparison to chemotherapy alone (33–45% vs. 15–32%) (36). Phase III trials are currently ongoing.
Of primary concern is the safety profile of chemotherapy plus checkpoint inhibitor combination therapy, as both are known to have toxicity-limiting effects. Preliminary data demonstrates a safety profile using combined chemotherapy with 5-FU and cisplatin in advanced GC which was manageable (32). The degree to which such combined therapies may be worth their toxicity profiles is debatable.
Radiation therapy has long been known to have potential effects on the immune system. The abscopal effect has been used to describe the phenomenon in which radiation therapy to principal lesion or region results in downsizing of remote lesions. This phenomenon has been described in numerous tumor types (37-39). The hypothesis for such an effect lies in the possibility of radiation-induced release of tumor-antigen which results in a system-wide antigen specific anti-tumor effect. Most descriptions of the effect have been case reports. For example, following the use of radiation therapy, reports have found an increase in new tumor antibodies formed as well as enhanced T-cell activation in addition to other reports describing remarkable response after combined radiation therapy and targeted immunotherapy (40). In addition to these descriptions, radiation therapy has been shown to recruit effector cells, induce proinflammatory cytokines, and enhance expression of MHC class I (41). All of these properties support its anti-tumor immune effect. However, dose and fractionation of radiation therapy likely play an integral role with regards to variation in response. Studies have tested radiation therapy in combination with vaccination therapy as well as adoptive T-cell therapy. These have demonstrated promising preclinical results in various cancer types (41). Gulley et al. demonstrated T-cell responses in 13 of 17 patients who received prostate-specific antigen (PSA) expressing vaccine in combination with radiotherapy (42). In this strategy, the index vaccination was done prior to treatment with radiation. However, with regards to the variety of immunotherapies described in this review, the timing and sequence of treatment would recognizably be dependent on type of combinatorial therapy.
Targeted-therapies can initiate concomitant release of tumor antigens after efficiently killing cancer cells, resulting a synergistic effect when combined with checkpoint inhibitors. A phase I trial provided evidence of both clinical activity and a manageable safety profile for an anti-PD-L1 antibody used either in combination with dabrafenib and trametinib in BRAF mutation-positive melanoma patients, in combination with trametinib in BRAF wild-type melanoma patients, or following trametinib in BRAF wild- type melanoma patients (43). The possible mechanism may be related to intratumoral T-cell accumulation and MHC I upregulation by MEK inhibition which could synergize with an anti-PDL1 agent to promote durable tumor regression (44).
Another strategy being implemented is the combination of immunotherapy with anti-VEGFR agents. Abnormal tumor angiogenesis can impede T effector cell infiltration into tumors, creating a hypoxic and acidic tumor microenvironment to the detriment of effector cells. Vascular normalizing by antiangiogenic agents provide a potential strategy to re-engineer the tumor-immune microenvironment and improve cancer immunotherapy (45). A pilot study showed a response rate of 40% when combining atezolizumab and bevacizumab in the treatment of metastatic renal cell carcinoma, which was greater than either single agent alone (46). As the above combinatorial strategies have shown early promise in many solid tumors, more clinical trials are needed to assess these types of strategies in GC to assess its practical use and toxicity profile.
Conclusions & limitations
Immunotherapy in GC has been a slowly progressing field of study. The field continues to grow evidenced by multiple ongoing clinical trials underway. ACT as a field has demonstrated convincing preclinical data in GC. Further study is warranted in the prospective randomized fashion to evaluate its use in combination with chemotherapy. Cancer vaccines in GC continue to be applied in clinical trials. Its role may be viewed as a weapon to enhance to anti-tumor immune response and may be the best applied in combination with checkpoint blockade therapy. As we have seen promising results in checkpoint blockade therapy in other cancers, the responses in GC are also convincing, substantiated by the recent FDA approval of pembrolizumab in advanced GC setting. However, future investigation is needed to identify which subset of patients may benefit most from a combinatorial strategy or if there are promising biomarkers which may predict response to therapy. These strategies aim to use therapies in the most advantageous methods. For example, effectiveness of immune therapies is often limited by the level of immune infiltration within the tumor microenvironment. Conceivably, a strategy to enhance the anti-tumor immune response by initial vaccination followed by treatment with checkpoint blockade therapy may work synergistically to elicit a more effective anti-tumor immune response. Figure 1 illustrates a simplistic depiction of this concept. Moreover, ameliorating toxicity profile associated with checkpoint blockade therapy, although improved with newer agents, is another area of interest. As use of immunotherapies in GC become more widely available, we must become more knowledgeable on managing the clinical side effects. Finally, with the eagerness of utilizing checkpoint blockade therapy in GC, we must be wary to not overindulge efforts into one mechanistic approach. As ACT and cancer vaccines have not shown as robust clinical responses as checkpoint blockade therapy, their utility may be underestimated. For example, response to checkpoint blockade therapy may be augmented by the ability of tumors mount immune cell infiltration. A strategy in which we may enhance immune cell infiltration in an antigen-specific manner using ACT followed by treatment with checkpoint blockade therapy to “release the breaks” off of the immune response may be a feasible approach to achieve the greatest anti-tumor effect. Furthermore, investigation into mechanisms of resistance for may bring light to the clinical value ACT and vaccination.

There are, however, limitations with regards to the use of immunotherapy in GC, as there are with other solid tumors. As there is variable immune cell infiltration in solid tumors, a tumor microenvironment enriched for immunosuppressive cells presents a major barrier which is not present in hematologic malignancies (47). Within the tumor microenvironment, various suppressive immune cells, such as Tregs, myeloid-derived suppressor cells (MDSCs), and tumor associated macrophages and neutrophils (TAMs and TANs) are considered to be obstacles against anti-tumor immunity. Another hurdle is the heterogeneity of solid tumor antigens available to be presented for an antigen specific response. It is difficult to identify a specific tumor antigen with both a robust response and high-level uniform expression. These shortcomings make it challenging to find the ideal target for antigen dependent immune therapies, perhaps best exemplified by CAR T-cell therapies (48).
Future directions
Immunotherapy in GC has seen great advances from its infancy. With the eagerness of investigators to clinically apply what has been demonstrated in recent breakthroughs in other cancer types, there is a need for the clever design and implementation of worthwhile clinical trials in GC based off of sound preclinical data. Of importance, more work is needed to identify biomarkers of response. Elegant work by Gopalakrishnan et al. demonstrate that response to PD-1 therapy can be modulated by the oral and gut microbiome in melanoma patients. Their work demonstrates an enhanced systemic anti-tumor immunity in patients responding to PD-1 therapy in which a favorable gut microbiome was present (49). Such striking observations demonstrate a new link between how systemic immune responses relate to local anti-tumor immunity. In addition to identifying biomarkers, more work is needed to identify mechanisms of resistance. The tumor stroma has major effects on tumor growth and is oftentimes underestimated in its contribution to tumor cell proliferation. The stroma is generally made up of nonmalignant cells which allow for continued tumor growth locally as well as in distant sites (50). These cells may be vital to the importance of anti-tumor immunity. For example, cancer-associated fibroblasts represent a cell type in the GC microenvironment which act synergistically with cancer cells as a driver to promote invasion and proliferation. These cells have been shown to promote angiogenesis in GC. While these cells are not immune cells, it remains to be elucidated how these cells respond to immune based therapies. Future study is required to better understand the effects on the components of the GC tumor microenvironment by immune based therapies, incorporating the “cross-talk” between malignant tumor cells, innate and adaptive immune cells, and stromal cells.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Zheng X, Song X, Shao Y, et al. Prognostic role of tumor-infiltrating lymphocytes in gastric cancer: a meta-analysis. Oncotarget 2017;8:57386-98. [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202-9. [Crossref] [PubMed]
- Lee K, Hwang H, Nam KT. Immune response and the tumor microenvironment: how they communicate to regulate gastric cancer. Gut Liver 2014;8:131-9. [Crossref] [PubMed]
- Jiang Y, Zhang Q, Hu Y, et al. ImmunoScore Signature: A Prognostic and Predictive Tool in Gastric Cancer. Ann Surg 2016. [Crossref] [PubMed]
- Kono K, Takahashi A, Ichihara F, et al. Prognostic significance of adoptive immunotherapy with tumor-associated lymphocytes in patients with advanced gastric cancer: a randomized trial. Clin Cancer Res 2002;8:1767-71. [PubMed]
- Sun Z, Shi L, Zhang H, et al. Immune modulation and safety profile of adoptive immunotherapy using expanded autologous activated lymphocytes against advanced cancer. Clin Immunol 2011;138:23-32. [Crossref] [PubMed]
- Zhang GQ, Zhao H, Wu JY, et al. Prolonged overall survival in gastric cancer patients after adoptive immunotherapy. World J Gastroenterol 2015;21:2777-85. [Crossref] [PubMed]
- Guo Y, Han W. Cytokine-induced killer (CIK) cells: from basic research to clinical translation. Chin J Cancer 2015;34:99-107. [Crossref] [PubMed]
- Jiang J, Xu N, Wu C, et al. Treatment of advanced gastric cancer by chemotherapy combined with autologous cytokine-induced killer cells. Anticancer Res 2006;26:2237-42. [PubMed]
- Liu K, Song G, Hu X, et al. A Positive Role of Cytokine-Induced Killer Cell Therapy on Gastric Cancer Therapy in a Chinese Population: A Systematic Meta-Analysis. Med Sci Monit 2015;21:3363-70. [Crossref] [PubMed]
- Zhang Q, Zhang Z, Peng M, et al. CAR-T cell therapy in gastrointestinal tumors and hepatic carcinoma: From bench to bedside. Oncoimmunology 2016;5:e1251539. [Crossref] [PubMed]
- Tran E, Ahmadzadeh M, Lu YC, et al. Immunogenicity of somatic mutations in human gastrointestinal cancers. Science 2015;350:1387-90. [Crossref] [PubMed]
- Tran E, Robbins PF, Lu YC, et al. T-Cell Transfer Therapy Targeting Mutant KRAS in Cancer. N Engl J Med 2016;375:2255-62. [Crossref] [PubMed]
- Tran E, Turcotte S, Gros A, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science 2014;344:641-5. [Crossref] [PubMed]
- Su S, Zou Z, Chen F, et al. CRISPR-Cas9-mediated disruption of PD-1 on human T cells for adoptive cellular therapies of EBV positive gastric cancer. Oncoimmunology 2016;6:e1249558. [Crossref] [PubMed]
- Masuzawa T, Fujiwara Y, Okada K, et al. Phase I/II study of S-1 plus cisplatin combined with peptide vaccines for human vascular endothelial growth factor receptor 1 and 2 in patients with advanced gastric cancer. Int J Oncol 2012;41:1297-304. [Crossref] [PubMed]
- Kono K, Takahashi A, Sugai H, et al. Dendritic cells pulsed with HER-2/neu-derived peptides can induce specific T-cell responses in patients with gastric cancer. Clin Cancer Res 2002;8:3394-400. [PubMed]
- Subhash VV, Yeo MS, Tan WL, et al. Strategies and Advancements in Harnessing the Immune System for Gastric Cancer Immunotherapy. J Immunol Res 2015;2015:308574.
- Ishikawa H, Imano M, Shiraishi O, et al. Phase I clinical trial of vaccination with LY6K-derived peptide in patients with advanced gastric cancer. Gastric Cancer 2014;17:173-80. [Crossref] [PubMed]
- Sadanaga N, Nagashima H, Mashino K, et al. Dendritic cell vaccination with MAGE peptide is a novel therapeutic approach for gastrointestinal carcinomas. Clin Cancer Res 2001;7:2277-84. [PubMed]
- Ott PA, Hu Z, Keskin DB, et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 2017;547:217-21. [Crossref] [PubMed]
- Sahin U, Derhovanessian E, Miller M, et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 2017;547:222-6. [Crossref] [PubMed]
- Chawla A, Philips AV, Alatrash G, et al. Immune checkpoints: A therapeutic target in triple negative breast cancer. Oncoimmunology 2014;3:e28325. [Crossref] [PubMed]
- Ito A, Kondo S, Tada K, et al. Clinical Development of Immune Checkpoint Inhibitors. Biomed Res Int 2015;2015:605478.
- Raufi AG, Klempner SJ. Immunotherapy for advanced gastric and esophageal cancer: preclinical rationale and ongoing clinical investigations. J Gastrointest Oncol 2015;6:561-9. [PubMed]
- Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer 2016;54:139-48. [Crossref] [PubMed]
- Schlößer HA, Drebber U, Kloth M, et al. Immune checkpoints programmed death 1 ligand 1 and cytotoxic T lymphocyte associated molecule 4 in gastric adenocarcinoma. Oncoimmunology 2015;5:e1100789. [Crossref] [PubMed]
- Thompson ED, Zahurak M, Murphy A, et al. Patterns of PD-L1 expression and CD8 T cell infiltration in gastric adenocarcinomas and associated immune stroma. Gut 2017;66:794-801. [Crossref] [PubMed]
- Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. [Crossref] [PubMed]
- Ralph C, Elkord E, Burt DJ, et al. Modulation of lymphocyte regulation for cancer therapy: a phase II trial of tremelimumab in advanced gastric and esophageal adenocarcinoma. Clin Cancer Res 2010;16:1662-72. [Crossref] [PubMed]
- Gu L, Chen M, Guo D, et al. PD-L1 and gastric cancer prognosis: A systematic review and meta-analysis. PLoS One 2017;12:e0182692. [Crossref] [PubMed]
- Fuchs CS, Denker AE, Tabernero J, et al. Pembrolizumab (MK-3475) for recurrent or metastatic gastric or gastroesophageal junction (GEJ) adenocarcinoma: Multicohort phase II KEYNOTE-059 study. J Clin Oncol 2016. [Epub ahead of print]. [Crossref]
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320-30. [Crossref] [PubMed]
- Bracci L, Schiavoni G, Sistigu A, et al. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ 2014;21:15-25. [Crossref] [PubMed]
- Pfirschke C, Engblom C, Rickelt S, et al. Immunogenic Chemotherapy Sensitizes Tumors to Checkpoint Blockade Therapy. Immunity 2016;44:343-54. [Crossref] [PubMed]
- Rizvi NA, Hellmann MD, Brahmer JR, et al. Nivolumab in Combination With Platinum-Based Doublet Chemotherapy for First-Line Treatment of Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:2969-79. [Crossref] [PubMed]
- Ehlers G, Fridman M. Abscopal effect of radiation in papillary adenocarcinoma. Br J Radiol 1973;46:220-2. [Crossref] [PubMed]
- Ohba K, Omagari K, Nakamura T, et al. Abscopal regression of hepatocellular carcinoma after radiotherapy for bone metastasis. Gut 1998;43:575-7. [Crossref] [PubMed]
- Wersäll PJ, Blomgren H, Pisa P, et al. Regression of non-irradiated metastases after extracranial stereotactic radiotherapy in metastatic renal cell carcinoma. Acta Oncol 2006;45:493-7. [Crossref] [PubMed]
- Kalbasi A, June CH, Haas N, et al. Radiation and immunotherapy: a synergistic combination. J Clin Invest 2013;123:2756-63. [Crossref] [PubMed]
- Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst 2013;105:256-65. [Crossref] [PubMed]
- Gulley JL, Arlen PM, Bastian A, et al. Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin Cancer Res 2005;11:3353-62. [Crossref] [PubMed]
- Ribas A, Butler M, Lutzky J, et al. Phase I study combining anti-PD-L1 (MEDI4736) with BRAF (dabrafenib) and/or MEK (trametinib) inhibitors in advanced melanoma. J Clin Oncol 2015;33:3003.
- Bendell JC, Kim TW, Goh BC, et al. Clinical activity and safety of cobimetinib (cobi) and atezolizumab in colorectal cancer (CRC). J Clin Oncol 2016;34:3502. [PubMed]
- Huang Y, Goel S, Duda DG, et al. Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Cancer Res 2013;73:2943-8. [Crossref] [PubMed]
- Wallin JJ, Bendell JC, Funke R, et al. Atezolizumab in combination with bevacizumab enhances antigen-specific T-cell migration in metastatic renal cell carcinoma. Nat Commun 2016;7:12624. [Crossref] [PubMed]
- Anderson KG, Stromnes IM, Greenberg PD. Obstacles Posed by the Tumor Microenvironment to T cell Activity: A Case for Synergistic Therapies. Cancer Cell 2017;31:311-25. [Crossref] [PubMed]
- Newick K, O'Brien S, Moon E, et al. CAR T Cell Therapy for Solid Tumors. Annu Rev Med 2017;68:139-52. [Crossref] [PubMed]
- Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Izar B, Joyce CE, Goff S, et al. Bidirectional cross talk between patient-derived melanoma and cancer-associated fibroblasts promotes invasion and proliferation. Pigment Cell Melanoma Res 2016;29:656-68. [Crossref] [PubMed]
Cite this article as: Chawla A, Wei J, Wang J. Current and developing immunotherapy in gastric adenocarcinoma. Art Surg 2017;1:9.


