
The pancreas from a surgical perspective: an illustrated overview
Introduction
The human pancreas is a solitary retroperitoneal organ. It has a “flat leaf” shape and lies obliquely across the upper abdomen at the level of the transpyloric plane. The typical adult human pancreas is 15–22 cm in length, 50–125 g in weight, and has a volume of approximately 73 cm3 (1). The pancreas has important exocrine and endocrine glandular functions. Its principal roles include the secretion of digestive enzymes and the regulation of serum glucose levels. The deep location of the pancreas and its proximity to other structures make pancreatic surgery challenging and high risk (2). This article aims to provide an overview of the aspects of the pancreas which are relevant to the surgeon/surgical trainee.
Anatomy
The pancreas is composed of a head, uncinate process, neck, and tail, and lies in the pararenal space. Its superior relations include the origin of the coeliac trunk, the common hepatic artery (CHA) and the splenic artery (2). Anteriorly, the stomach, lesser sac (omental bursa) and transverse mesocolon can be found. Posteriorly are the aorta, inferior vena cava (IVC), portal vein (PV), and body of the second lumbar vertebra (L2) (2). The head, the widest part; is disc-shaped and “wrapped” by the inner curve created by the first three parts of the duodenum, to which it is connected via connective tissue. The head lies lateral to (to the right of) the superior mesenteric artery (SMA) and the superior mesenteric vein (SMV). The inferior extension of the head is the uncinate process. This is a hook shaped continuation of the inferomedial part of the head. It sits within the curve of the fourth part of the duodenum. The SMV and, occasionally, the SMA descend on its anterior surface. The neck of the pancreas, which connects the head to the tail, overlies the superior mesenteric vessels, which from a grove in its posterior surface (2). The body lies to the left of the superior mesenteric vessels. Its anterior surface is covered by peritoneum which forms part of the posterior surface of the lesser sac. The body is located anterior to the aorta and protrudes superiorly towards the spleen. The splenic artery follows the course of the body and creates a grove in its posterior and superior surface (2). The tail is extra-peritoneal and lies in close proximity to the splenic hilum. It is contained within the splenorenal ligament.
The head and uncinate process receive their blood supply from the superior (SPDA) and inferior (IPDA) pancreaticoduodenal arteries, which each contribute to the anterior and posterior pancreaticoduodenal arcades (Figure 1) (2). The SPDA is a branch of the gastroduodenal artery. This derives from the CHA, a branch of the coeliac trunk. The IPDA arises from the SMA. Thus, the head and uncinate process receive blood from both embryological foregut and embryological midgut sources (2). The body and tail receive their blood supply from numerous branches of the splenic artery (3). The neck is a watershed area between these two vascular systems and venous drainage is via the portal system. The head and neck are mostly drained via the superior mesenteric branches of the PV. The remainder of the pancreas is drained by short, fragile branches of the splenic vein. The splenic vein passes posteriorly to the body where it joins the SMV to form the PV behind the neck (2).
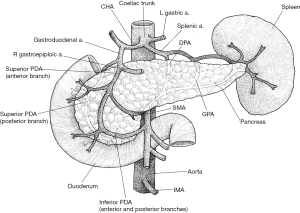
The pancreas is drained by a lymphatic network that largely follows that of the arterial supply. The vessels draining the head empty into the pyloric nodes, and the vessels draining the body and tail drain into the pancreatosplenic nodes. These ultimately drain into the superior mesenteric and coeliac nodes. The pancreas receives rich autonomic innervation as separate signalling pathways regulate the exocrine and endocrine functions. The parasympathetic component is received via fibres from the tenth cranial nerves, the vagus nerves (4). Sympathetic innervation is from the lesser splanchnic nerves, which originate from the fifth to the twelfth thoracic vertebral levels (4).
Within the pancreas is a system of ducts (Figure 2) of which there are known anatomical variants. Typically, the main pancreatic duct (of Wirsung) travels the entire ventral length of the pancreas from the tail to the bile duct, where the ampulla of Vater is formed. This opens into the second part of the duodenum at the major duodenal papilla (1). The passing of secretions is controlled by the sphincter of Oddi, a smooth muscle sphincter which also prevents the reflux of enteral content into the ampulla. Often there is a dorsal accessory duct (of Santorini) which has a confluence with the ventral duct at the genu. The accessory duct usually opens into the second part of the duodenum at the minor duodenal papilla (separate from the ampulla) (1).
Development
In order to understand how the pancreas develops it is reasonable to first consider how the duodenum develops (Figure 3). By the fourth gestational week, the primitive gut tube has formed into a gastrointestinal tract composed of the foregut, midgut and hindgut. Shortly after this, the proximal duodenum develops from the caudal foregut whilst the distal part originates from the cranial midgut. Hence, it is supplied by arteries of both coeliac artery and SMA origin. The junction of the two parts is found just distal to the ampulla of Vater. The “C-shape” of the duodenum initially projects anteriorly but, once the stomach has rotated, this moves to the right and presses against the posterior abdominal wall, becoming retroperitoneal.
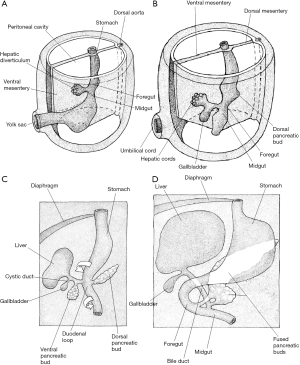
Information regarding the development of the human pancreas is limited and most of our understanding regarding the critical steps is extrapolated from chick and mouse models (1). Human pancreas formation is first evident at the twenty-sixth gestational day (1). It derives from a dorsal bud and two ventral buds which form on either side of the distal foregut endoderm (5). The left ventral bud regresses whilst the right bud migrates posteriorly where is fuses with the posterior bud upon gut rotation at around 6 weeks of gestation (Figure 4) (1). The dorsal bud forms the majority of the pancreas, including the superior head, neck, body, and tail (6). The inferior portion of the head is derived from the right ventral bud (6).
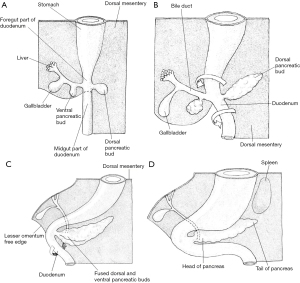
Prior to the appearance of the dorsal bud, the dorsal embryonic endoderm is in contact with the notochord. This is a midline structure which acts as a primitive axial skeleton prior to the formation of other elements (7). This, it is hypothesised, prevents the expression of sonic hedgehog (SHH) protein which provides permissive signals for pancreas development (5). Transient contact between the notochord and endoderm is disrupted by the paired dorsal aortae which fuse with the surrounding mesoderm at around day 30 of gestation. The splanchnic mesoderm separates the dorsal bud from the fused dorsal aortae at around day 36. Subsequently, the pancreatic buds extend into the adjacent mesenchyme (8). Growth and proliferation of the pancreatic epithelial cells is stimulated by fibroblast growth factors 7 and 10, which are provided by the mesenchyme itself (8). Starting at around day 45 of gestation, active branching morphogenesis begins within the pancreatic epithelium (1) and the epithelium is enveloped by a dense peripancreatic mesenchyme. At around week 7, the typical pancreatic lobular pattern becomes apparent (9).
Hormone-expressing endocrine cells are not present in the human embryonic pancreas until 8 weeks of gestation (10). By week 10, these are beginning to form into clusters and by week 14 they resemble islets with a distinct blood supply (11). Less is known regarding the development of the exocrine pancreas. It is thought that precursor acinar cells form from the pancreatic epithelium from week 8 (5). Several digestive enzymes show a drastic increase in their expression from week 11 but amylase is not detected until week 23 (5). Messenger ribonucleic acid expression of ductal cell markers has been detected from week 11 but ductal cell differentiation has not been clearly defined (1). It is thought this occurs between weeks 24 and 32 (6).
Anatomical variants
Congenital abnormalities and anatomical variants of the pancreas are not uncommon (Figure 5). Whilst the majority are only detected incidentally, some are clinically relevant. Pancreas divisum is the most common and is thought to affect over 10% of the population (12). This represents a variation in ductal anatomy and occurs when the dorsal and ventral ducts do not fuse and the dorsal duct opens into the duodenum via the minor papilla. As such, there is no communication between the main duct and the accessory duct (Figure 5A). The term dominant dorsal duct syndrome is also used to describe this variant. Figure 5A illustrates “complete” pancreas divisum but an “incomplete” version is also possible where there is some communication between the main duct and the accessory duct via small channels. Additionally, “reverse” pancreas divisum may occur where the main duct continues to the major papilla but the accessory duct does not communicate with the main duct. Whilst most patients with pancreas divisum are asymptomatic, some may experience abdominal pain or develop recurrent pancreatitis. In such cases, endoscopic retrograde cholangiopancreatography (ERCP) and sphincterotomy of the minor papilla may be indicated (13).
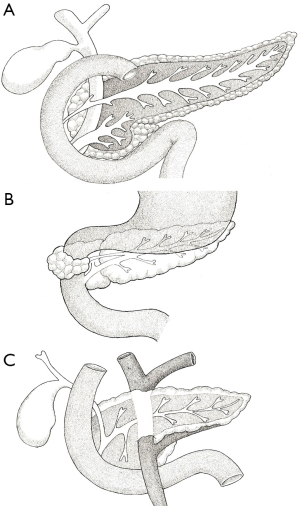
An annular pancreas (Figure 5B) is thought to affect around 1 in 20,000 individuals. In this anomaly, a portion of pancreatic tissue wraps around the second part of the duodenum (12). This is thought to be the result of adherence of the right ventral bud to the duodenum, or failure of the left ventral bud to regress (14). Whilst usually asymptomatic, early signs can include failure-to-thrive and feeding intolerance. In adults who go on to develop pancreatitis, the subsequent scar tissue formed can result in duodenal obstruction. This can produce the “double bubble” sign seen on abdominal radiographs, which is also observed in duodenal atresia and intestinal malrotation (15). In such cases, surgical intervention may be necessary.
A circumportal pancreas (Figure 5C) results when pancreatic tissue envelops the PV or SMV. It is found in 1.1–2.5% of individuals and is thought to result from abnormal fusion of the dorsal and ventral buds. Again, patients are usually asymptomatic but this variation must be considered when planning any surgical resection as it may increase the risk of certain complications such as postoperative pancreatic fistula (16). Other anatomical variations are extremely rare. Absence of the body and tail is known as dorsal pancreas agenesis and results from the developmental failure of the dorsal bud. Most patients are asymptomatic but some may present with abdominal pain (15). Other abnormalities relate to the presence of ectopic pancreatic tissue. The most common sites are the stomach and duodenum (17).
Exocrine function
An exocrine gland secretes a substance into a ductal system or onto an epithelial surface, whereas an endocrine gland secretes a substance directly into the bloodstream. The majority of pancreatic tissue, over 85%, is exocrine in nature and is composed of units called acini, which are made up of acinar cells (Figure 6) (18). These cells, under the tight regulation of the neuroendocrine system, synthesise and secrete enzymes which aid in the digestion of carbohydrates, proteins and fats (19). These enzymes include trypsinogen, chymotrypsinogen, elastase, carboxypeptidase, pancreatic lipase, nucleases, and amylase. Each acinar bundle is in direct communication with the pancreatic ductal system (18). Centroacinar cells are the most peripheral exocrine cells; they partially cover the apical surface of the acinar cells. These connect to a system of intercalated ducts which form intra- and interlobular ducts (18). These ultimately collect into the main pancreatic duct.
The secretions of the acinar cells combine with an alkaline, isotonic, bicarbonate-rich solution produced by the pancreatic ductal epithelial cells to form pancreatic juice (20). This process is principally regulated by acetylcholine, released from vagal nerve endings, and cholecystokinin, an intestinal hormone. Secretin and vasoactive intestinal peptide (VIP) are also involved in this process. Cholecystokinin and secretin are released by the duodenal epithelial cells in response to the luminal presence of acidic chyme (21). The bicarbonate-rich nature of the pancreatic juice ensures optimum enzyme function within the digestive tract. The rate of secretion, which itself is hormone-regulated, is markedly increased during mealtimes. Twenty-four-hour total output is between 2 and 3 litres, and this includes approximately 20 g of digestive enzymes (18).
Exocrine tumours
Pancreatic cancers can be broadly categorised into two groups; those arising in exocrine cells (the vast majority) and those which arise in endocrine cells [collectively called pancreatic neuroendocrine tumours (PanNETs)]. Over 90% of exocrine tumours are pancreatic ductal adenocarcinomas (PDACs) (22). These, as the name suggests, arise from the ductal epithelial cells. Since the vast majority of pancreatic cancers are PDACs, the two terms are often used synonymously. Approximately 5% of exocrine tumours arise from the acinar cells and are termed acinar cell carcinomas (23). Other rare exocrine cancers include cystadenocarcinomas, pancreaticoblastomas, adenosquamous carcinomas, signet ring carcinomas, and hepatoid carcinomas. Others have been described but these are exceedingly rare.
PDAC prognosis is poor due to the lack of early diagnostic markers, delayed detection, variable genetic profiles, and rapid metastasis (24). Over three quarters of patients present with unresectable disease (24). These patients are managed by oncologists or palliative care physicians. Those with a periampullary tumour that obstructs the flow of bile may require ERCP. For fit patients with early disease, curative intent surgical resection is recommended. This may be in the form of pancreatoduodenectomy (PD, also known as Whipple’s procedure) for those with disease affecting the head of the pancreas, or distal pancreatectomy (DP) for those with disease affecting the body or tail. Some patients may require total pancreatectomy. PD refers to resection of the pancreatic head, duodenum, gall bladder and bile duct (along with most of the common hepatic duct). Following the resection, it is necessary to fashion a pancreatojejunostomy (or pancreatogastrostomy), hepaticojejunostomy and gastrojejunostomy in order to restore intestinal continuity and ensure both bile and pancreatic juice reach the gut. Whilst selected units perform this procedure laparoscopically or robotically, an open procedure remains the standard of care (25).
Depending on the site and stage of the tumour, DP can be performed with or without splenic preservation. Both the spleen-preserving and spleen-resecting approaches can be performed laparoscopically where the appropriate expertise is available (26). All forms of pancreatic resection are high risk and associated with significant morbidity. An especially feared complication is postoperative pancreatic fistula which, when biochemical leaks are excluded, affects up to a quarter of resections (27). This occurs when there is pancreatic juice leakage from a surgically exfoliated surface and/or anastomotic stump. This complication is challenging to manage and is associated with additional morbidity (28). Overall morbidity following PD is in the region of 50%. Sadly, even if recovery is uneventful, most patients develop recurrent disease and few achieve long term survival.
Endocrine function
The endocrine function is provided by groups of cells known as the pancreatic islets or islets of Langerhans (Figure 6). These take their name from the German pathologist, Paul Langerhans, and are scattered throughout the parenchyma (29,30). A typical human pancreas has 3.2–14.8 million islets (30,31). Whilst each can contain up to a few thousand endocrine cells, the islets themselves make up only 2% of the total pancreatic tissue mass (31). An islet usually contains a central core of beta cells surrounded by a ring of alpha cells. Beta cells secrete the peptide hormone insulin which stimulates glucose uptake by the tissues. Alpha cells secrete glucagon, another peptide hormone, which counteracts insulin and increases serum glucose concentration (32). In healthy individuals, the production of insulin and glucagon are mediated by negative feedback mechanisms (32).
Alpha and beta cells make up around 90% of islet cells (29). The remaining 10% include delta cells, pancreatic polypeptide (PP) cells, epsilon cells, and others. Delta cells release somatostatin in response to acetylcholine, glutamate, urocortin-3, ghrelin, and high glucose concentrations (33). Also known as growth hormone inhibiting hormone, somatostatin is a cyclic peptide which is known for its strong regulatory effects on various gastrointestinal and central nervous system functions (33). It is a negative regulator of both insulin and glucagon (34). PP cells, also known as F or gamma cells, comprise 1–2% of islet cells (29). They secrete PP, an inhibitor of glucagon when serum glucose concentrations are low (35), and are thought to have a role in satiety (36). Ghrelin, produced by epsilon cells, inhibits the secretion of insulin and induces hunger.
Endocrine tumours
PanNETs arise from islet cells. They were previously known as islet cell tumours. They represent less than 2% of all pancreatic tumours and tend to present in the sixth or seventh decade of life with a slight male predominance (37). They represent a behaviourally diverse group of lesions and may be sporadic or the result of hereditary syndromes, such as von Hippel-Lindau syndrome, neurofibromatosis or multiple endocrine neoplasia type one (37). The majority are non-functioning but some can produce the hormone synthesised by their precursor cell. This can result in a number of different symptoms/signs, e.g., an insulinoma may present with hypoglycaemia and a VIPoma may present with watery diarrhoea and hypokalaemia (37). The signs and symptoms of non-functioning PanNETS are mass-related as in exocrine tumours e.g., obstructive jaundice. The most common hormone-secreting tumours are insulinomas, followed by gastrinomas. VIPomas, glucagonomas and somatostatinomas are very rare (38). Although PanNETs are generally slow-growing, most patients present with metastases as they are usually asymptomatic whilst the disease remains resectable (39). In those with early disease and an appropriate performance status, surgical resection remains the cornerstone of treatment since it is the only curative-intent option.
Other surgical pathology
Aside from malignancy, a range of other pathologies can affect the pancreas. These include numerous benign lesions and medical disorders which will not be covered. Acute pancreatitis is an inflammatory condition which typically presents with acute onset epigastric pain. This may be associated with nausea, vomiting, and/or a change in bowel habit. A diagnosis is usually made when the serum amylase or lipase is more than three times the upper limit of normal, or following computed tomography (CT). Approximately half of United Kingdom (UK) cases are the result of gallstones migrating from the gallbladder to the bile duct (choledocholithiasis) where they can impede the flow of pancreatic juice (40). The enzymes then induce inflammation within the pancreas itself. The second most common cause of acute pancreatitis in the UK is alcohol consumption (40). This results when pancreatic acinar cells metabolise ethanol into toxic substances which cause injury to the pancreatic ducts. The parenchyma is then exposed to digestive enzymes and an inflammatory process ensues. Acute pancreatitis can also be caused by medical interventions which traumatise the ampulla, such as ERCP (40). Less common causes include hypertriglyceridaemia, medications, viral infections, trauma and autoimmune conditions. Acute pancreatitis is usually self-limiting but patients can become very unwell and require multi-organ support. Unwell patients with choledocholithiasis may require urgent ERCP and sphincterotomy. Complications resulting from acute pancreatitis, such as adult respiratory distress syndrome (ARDS) and intra-abdominal sepsis, can be life threatening. Severe pancreatitis can result in necrosis to the pancreatic and/or retroperitoneal tissues and an abscess can form. Historically, an open necrosectomy was performed but outcomes were known to be poor so this technique is now reserved for exceptional circumstances. Nowadays, it is more common to attempt to drain areas of necrosis using endoscopic or radiologic-guided approaches.
Chronic pancreatitis is a syndrome characterised by chronic progressive pancreatic inflammation, fibrosis and scarring (41). This can result in tissue loss and affect pancreatic function. Current theories suggest that a cascade of steps towards chronic inflammation is triggered when there is ongoing injury to the pancreas via oxidative stress or repeated episodes of acute inflammation (41). Risk factors include continued exposure to alcohol, smoking, a genetic predisposition, and ductal obstruction (41). Patients usually present with epigastric pain although they may not have significantly raised serum amylase/lipase levels if they have undergone significant pancreatic tissue loss. Patients become malnourished and experience significant weight loss. Management focuses on patient education, counselling regarding alcohol consumption, a multi-disciplinary approach to pain management, and medical treatment for the resulting pancreatic exocrine insufficiency or diabetes mellitus (42). Chronic pancreatitis is a known risk factor for the development of PDAC (43).
Acknowledgments
We would like to thank John Peter Ovens for providing the illustrations.
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://aos.amegroups.com/article/view/10.21037/aos-21-2/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pan FC, Brissova M. Pancreas development in humans. Curr Opin Endocrinol Diabetes Obes 2014;21:77-82. [Crossref] [PubMed]
- Mahadevan V. Anatomy of the pancreas and spleen. Surgery (Oxford) 2019;37:297-301. [Crossref]
- Covantev S, Mazuruc N, Belic O. The Arterial Supply of the Distal Part of the Pancreas. Surg Res Pract 2019;2019:5804047. [Crossref] [PubMed]
- Li W, Yu G, Liu Y, et al. Intrapancreatic Ganglia and Neural Regulation of Pancreatic Endocrine Secretion. Front Neurosci 2019;13:21. [Crossref] [PubMed]
- Jennings RE, Berry AA, Strutt JP, et al. Human pancreas development. Development 2015;142:3126-37. [Crossref] [PubMed]
- Adda G, Hannoun L, Loygue J. Development of the human pancreas: variations and pathology. A tentative classification. Anat Clin 1984;5:275-83. [Crossref] [PubMed]
- Stemple DL. Structure and function of the notochord: an essential organ for chordate development. Development 2005;132:2503-12. [Crossref] [PubMed]
- Ye F, Duvillié B, Scharfmann R. Fibroblast growth factors 7 and 10 are expressed in the human embryonic pancreatic mesenchyme and promote the proliferation of embryonic pancreatic epithelial cells. Diabetologia 2005;48:277-81. [Crossref] [PubMed]
- Pan FC, Wright C. Pancreas organogenesis: from bud to plexus to gland. Dev Dyn 2011;240:530-65. [Crossref] [PubMed]
- Piper K, Brickwood S, Turnpenny LW, et al. Beta cell differentiation during early human pancreas development. J Endocrinol 2004;181:11-23. [Crossref] [PubMed]
- Meier JJ, Köhler CU, Alkhatib B, et al. Beta-cell development and turnover during prenatal life in humans. Eur J Endocrinol 2010;162:559-68. [Crossref] [PubMed]
- Yu J, Turner MA, Fulcher AS, et al. Congenital anomalies and normal variants of the pancreaticobiliary tract and the pancreas in adults: part 2, Pancreatic duct and pancreas. AJR Am J Roentgenol 2006;187:1544-53. [Crossref] [PubMed]
- Ferri V, Vicente E, Quijano Y, et al. Diagnosis and treatment of pancreas divisum: A literature review. Hepatobiliary Pancreat Dis Int 2019;18:332-6. [Crossref] [PubMed]
- Tadokoro H, Takase M, Nobukawa B. Development and congenital anomalies of the pancreas. Anat Res Int 2011;2011:351217. [Crossref] [PubMed]
- Mortelé KJ, Rocha TC, Streeter JL, et al. Multimodality imaging of pancreatic and biliary congenital anomalies. Radiographics 2006;26:715-31. [Crossref] [PubMed]
- Connelly TM, Sakala M, Tappouni R. Circumportal pancreas: a review of the literature and image findings. Surg Radiol Anat 2015;37:431-7. [Crossref] [PubMed]
- Rezvani M, Menias C, Sandrasegaran K, et al. Heterotopic Pancreas: Histopathologic Features, Imaging Findings, and Complications. Radiographics 2017;37:484-99. [Crossref] [PubMed]
- Atkinson MA, Campbell-Thompson M, Kusmartseva I, et al. Organisation of the human pancreas in health and in diabetes. Diabetologia 2020;63:1966-73. [Crossref] [PubMed]
- Matsuda Y. Age-related morphological changes in the pancreas and their association with pancreatic carcinogenesis. Pathol Int 2019;69:450-62. [Crossref] [PubMed]
- Chandra R, Liddle RA. Modulation of pancreatic exocrine and endocrine secretion. Curr Opin Gastroenterol 2013;29:517-22. [Crossref] [PubMed]
- Pandiri AR. Overview of exocrine pancreatic pathobiology. Toxicol Pathol 2014;42:207-16. [Crossref] [PubMed]
- Kleeff J, Korc M, Apte M, et al. Pancreatic cancer. Nat Rev Dis Primers 2016;2:16022. [Crossref] [PubMed]
- Al-Hader A, Al-Rohil RN, Han H, et al. Pancreatic acinar cell carcinoma: A review on molecular profiling of patient tumors. World J Gastroenterol 2017;23:7945-51. [Crossref] [PubMed]
- Sarantis P, Koustas E, Papadimitropoulou A, et al. Pancreatic ductal adenocarcinoma: Treatment hurdles, tumor microenvironment and immunotherapy. World J Gastrointest Oncol 2020;12:173-81. [Crossref] [PubMed]
- Nassour I, Wang SC, Porembka MR, et al. Robotic Versus Laparoscopic Pancreaticoduodenectomy: a NSQIP Analysis. J Gastrointest Surg 2017;21:1784-92. [Crossref] [PubMed]
- Han HS, Yoon YS, Kwon SU, et al. Laparoscopic spleen preserving distal pancreatectomy. J Vis Surg 2016;2:146. [Crossref] [PubMed]
- Williamsson C, Stenvall K, Wennerblom J, et al. Predictive Factors for Postoperative Pancreatic Fistula-A Swedish Nationwide Register-Based Study. World J Surg 2020;44:4207-13. [Crossref] [PubMed]
- Nahm CB, Connor SJ, Samra JS, et al. Postoperative pancreatic fistula: a review of traditional and emerging concepts. Clin Exp Gastroenterol 2018;11:105-18. [Crossref] [PubMed]
- Da Silva Xavier G. The Cells of the Islets of Langerhans. J Clin Med 2018;7:54. [Crossref] [PubMed]
- Kim A, Miller K, Jo J, et al. Islet architecture: A comparative study. Islets 2009;1:129-36. [Crossref] [PubMed]
- Ionescu-Tirgoviste C, Gagniuc PA, Gubceac E, et al. A 3D map of the islet routes throughout the healthy human pancreas. Sci Rep 2015;5:14634. [Crossref] [PubMed]
- Habegger KM, Heppner KM, Geary N, et al. The metabolic actions of glucagon revisited. Nat Rev Endocrinol 2010;6:689-97. [Crossref] [PubMed]
- Arrojo E, Drigo R, Jacob S, García-Prieto CF, et al. Structural basis for delta cell paracrine regulation in pancreatic islets. Nat Commun 2019;10:3700. [Crossref] [PubMed]
- Kailey B, van de Bunt M, Cheley S, et al. SSTR2 is the functionally dominant somatostatin receptor in human pancreatic β- and α-cells. Am J Physiol Endocrinol Metab 2012;303:E1107-16. [Crossref] [PubMed]
- Aragón F, Karaca M, Novials A, et al. Pancreatic polypeptide regulates glucagon release through PPYR1 receptors expressed in mouse and human alpha-cells. Biochim Biophys Acta 2015;1850:343-51. [Crossref] [PubMed]
- Tan TM, Field BC, Minnion JS, et al. Pharmacokinetics, adverse effects and tolerability of a novel analogue of human pancreatic polypeptide, PP 1420. Br J Clin Pharmacol 2012;73:232-9. [Crossref] [PubMed]
- Kelgiorgi D, Dervenis C. Pancreatic neuroendocrine tumors: the basics, the gray zone, and the target. F1000Res 2017;6:663. [Crossref] [PubMed]
- Halfdanarson TR, Rubin J, Farnell MB, et al. Pancreatic endocrine neoplasms: epidemiology and prognosis of pancreatic endocrine tumors. Endocr Relat Cancer 2008;15:409-27. [Crossref] [PubMed]
- Schimmack S, Svejda B, Lawrence B, et al. The diversity and commonalities of gastroenteropancreatic neuroendocrine tumors. Langenbecks Arch Surg 2011;396:273-98. [Crossref] [PubMed]
- Shah AP, Mourad MM, Bramhall SR. Acute pancreatitis: current perspectives on diagnosis and management. J Inflamm Res 2018;11:77-85. [Crossref] [PubMed]
- Pham A, Forsmark C. Chronic pancreatitis: review and update of etiology, risk factors, and management. F1000Res 2018;7:eF1000 Faculty Rev-607.
- Forsmark CE. Management of chronic pancreatitis. Gastroenterology 2013;144:1282-91.e3. [Crossref] [PubMed]
- Bond-Smith G, Banga N, Hammond TM, et al. Pancreatic adenocarcinoma. BMJ 2012;344:e2476. [Crossref] [PubMed]
Cite this article as: Russell TB, Aroori S. The pancreas from a surgical perspective: an illustrated overview. Art Surg 2022;6:1.




